Research Article |
|
Corresponding author: Volker W. Framenau ( volker.framenau@murdoch.edu.au ) Academic editor: Danilo Harms
© 2022 Volker W. Framenau, Pedro de S. Castanheira.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Framenau VW, Castanheira PS (2022) A new genus of Australian orb-weaving spider with extreme sexual size dimorphism (Araneae, Araneidae). Zoosystematics and Evolution 98(1): 139-149. https://doi.org/10.3897/zse.98.82649
|
Abstract
The new Australian orb-weaving spider genus Mangrovia in the family Araneidae Clerck, 1757 is described. It is characterised by extreme sexual size-dimorphism (eSSD) with females (total length 8–10 mm) ca. 3 to 5 times larger than males (2.5–3 mm). Whilst Mangrovia shares with the informal Australian ‘backobourkiine’ clade a single seta on the male pedipalp patella, the genus is probably more closely related to the ‘zealaraneines’ or associated genera. In addition to eSSD and the single patellar spine, the genus is characterised by a distinct subterminal embolus branch in males. The new genus includes two species: the type species Mangrovia albida (L. Koch, 1871) comb. nov. (= Epeira fastidiosa Keyserling, 1887, new syn.) from Queensland and Mangrovia occidentalis sp. nov. from Western Australia. Both species are apparently coastal and occur in mangroves, but also in riparian woodland. Spiders were found resting in rolled-up leaves adjacent to their orb-web.
Key Words
Backobourkiines, new combination, new species, systematics, taxonomy, zealaraneines
Introduction
Extreme sexual size dimorphism (eSSD), a phenomenon where one sex – generally the female – is at least twice as big as the other, is not a common phenomenon in spiders and mostly expressed in orb-weaving taxa (family Araneidae Clerck, 1757, incl. Nephilinae Simon, 1894 and Phonognathinae Simon, 1894) and comb-footed spiders (family Theridiidae) (
The incidence of eSSD is low within the traditional Araneinae Clerck, 1757 (sensu
A second well-supported group including Australian orb-weavers are the ‘zealaraneines’, which largely include New Zealand genera such as Colaranea Court & Forster, 1988, Cryptaranea Court & Forster, 1988 and Zealaranea Court & Forster, 1988, but also the Australian species Araneus albotriangulus (Keyserling, 1887) and A. talipedatus (Keyserling, 1887), both misplaced at the genus level and representing new genera (
Our current revision of Australian orb-weaving spiders recovered a novel case of eSSD. Females of Araneus albidus (L. Koch, 1871), a species originally described based on a female only, are approximately three to five times larger than males. As in the dehaani-group, eSSD appears to act at the genus level, as a second undescribed species very similar to A. albidus is also extremely size-dimorphic. The male pedipalps of A. albidus (and the undescribed species) have only one patellar spine suggesting close affinities with the backobourkiines. However, other genitalic characters, for example the shape of the median apophysis of the male pedipalp, do not match any of the genera currently recognised in this group and these species may therefore be a zealaraneine, or not belong to any of these two well-supported indigenous Australo-Oriental groups.
The aim of this study is to describe these two species in a new genus as a working hypothesis for future phylogenetic analyses of Australian Araneidae, specifically the species of the backobourkiines and zealaraneines sensu
Materials and methods
Descriptions and terminology follow recent publications on Australian orb-weaving spiders (e.g.
Male pedipalps were expanded by alternately submerging them for ca. 20 min in warm 10%KOH and distilled water until maximum expansion was reached. Female genitalia were dissected and then cleared in warm 10%KOH for 20 mins and transferred into lactic acid on a microscopic slide under a coverglass to further clear internal features for imaging. Measurements are given in millimetres taken at an accuracy of one tenth of a millimetre, except for eye and labium sizes measured at a hundredth of a millimetre.
Images of preserved specimens were taken in different focal planes with a Nikon D300 digital SLR camera attached to a Leica M16A stereomicroscope and combined with Auto Montage (vers. 5.02) software from Syncroscopy to increase depth of field. We used 2 Nikon R1C1 wireless speedlights instead of fibre optics to illuminate the exposures. The latter were used as guide-light for focusing. Microscopic images of cleared epigynes and expanded pedipalps were taken in different focal planes (ca. 20–30 images) on a Leica DMC4500 digital camera mounted to a Leica M205C stereomicroscope and combined using the Leica Application Suite X, v. 3.6.0.20104. All photos were edited and mounted in the software Photoshop CC 2020.
Maps were compiled in the software package QGis 3.22.3 ‘Białowieża (https://qgis.org/en/site/; accessed 20 February 2022). Geographic coordinates were extracted directly from original labels or the registration data as provided by the museums. When no detailed geographic information was available, localities were estimated based on Google Earth v. 7.3.4.8248 (64-bit) (https://earth.google.com/web/; accessed 21 February 2022).
Abbreviations
Collections
Morphology
AME, ALE anterior median (lateral) eyes
PME, PLE posterior median (lateral) eyes
Results
Taxonomy
Class Arachnida Cuvier, 1812
Order Araneae Clerck, 1757
Family Araneidae Clerck, 1757
Mangrovia gen. nov.
Type-species
Epeira albida L. Koch, 1871, designated here.
Etymology
The genus-group name is derived from the general habitat preferences of the two species, which are often found in coastal mangroves and woodlands. The gender is feminine.
Diagnosis
Within an Australian context, Mangrovia gen. nov. males have only a single patellar spine on the pedipalp, a character considered a putative synapomorphy of the backobourkiines. However, Mangrovia gen nov. differ considerably from members of the backobourkiines by somatic and genital morphology. Both species in the genus display eSSD with females about 3–5 times larger than males, but eSSD is absent in the backobourkiines (with the exception of B. collina and species in the dehaani-group (sensu
Mangrovia gen. nov. differs from the members of the zealaraneines as currently known (see Introduction section) by its eSSD, absent in any known zealaraneines, and the presence of only one patellar spine on the male pedipalp, whereas there are always two in zealaraneines (
Outside the backobourkiines and zealaraneines, Mangrovia gen. nov. appears most similar to species of Neoscona (
Description
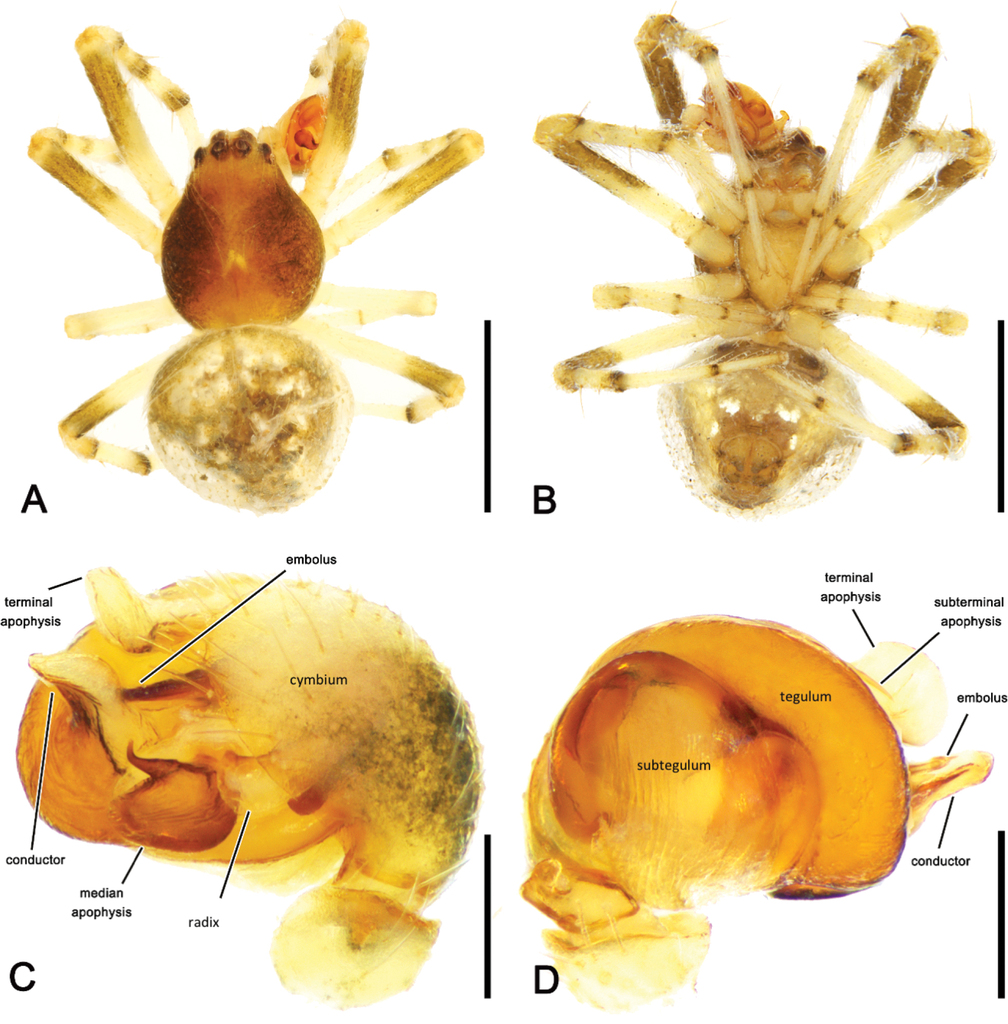
Small to medium-sized orb-weaving spiders with eSSD (TL males ca. 2.5–3 mm, females ca. 8–10 mm). Carapace (Figs
Composition
Mangrovia albida (L. Koch, 1871) comb. nov. and Mangrovia occidentalis sp. nov.
Distribution
Australia (Queensland and Western Australia) (Figs
Mangrovia albida , comb. nov.
Epeira albida
L.
Araneus albidus
(L. Koch).-
Epeira fastidiosa
Araneus fastidiosus
(Keyserling).-
Type material
Holotype
of Epeira albida L.
Holotype
of Epeira fastidiosa Keyserling, 1887: male Rockingham, (23°23'S, 150°30'E, Queensland, Australia) (
Other material examined
Australia: Queensland: 1 female, Brisbane, 27°28'S, 153°01'E (
Diagnosis
Males M. albida comb. nov. can be separated from M. occidentalis sp. nov. by subtle differences in key pedipalp sclerites, specifically the conductor is less elongate (Figs
Description
Male
(based on
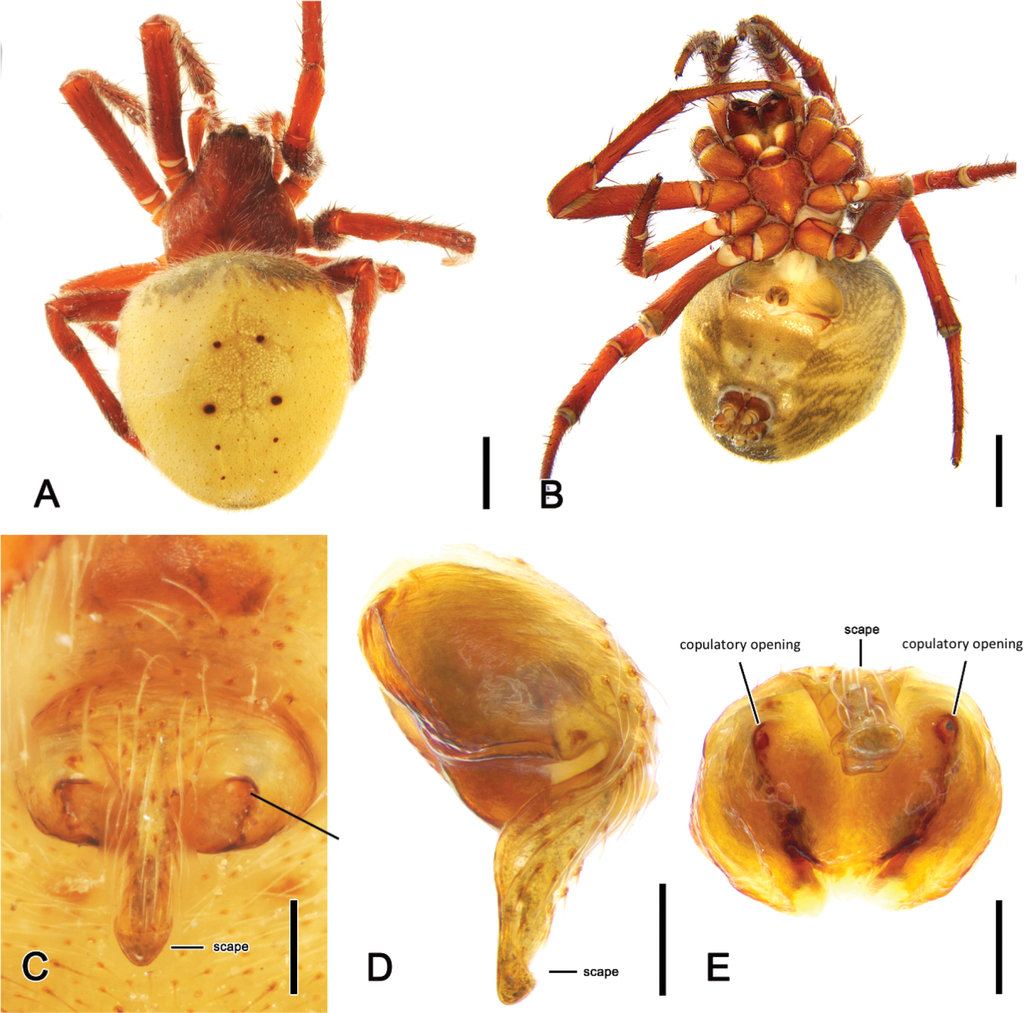
Female
(based on
Variation
Total length males 2.7–3.0 (n = 3), females 8.3–9.7 (n = 5). There is very little colour variation in both males and females, although the folium pattern in males can be very distinct.
Remarks
Habitat preferences and life history
Habitat descriptions found on labels with museum specimens include ‘mangroves’ and ‘riparian’, where spiders were found in rolled leaves near the orb-web. Mature spiders were found between December and April.
Mangrovia occidentalis sp. nov.
Type material
Holotype
male, Cape Range National Park, Yardie Creek (22°20'S, 113°48'E, Western Australia, Australia), 7 July 1987, B. Y. Main (
Etymology
The specific epithet is a Latin adjective in apposition – occidentalis – meaning western, and it refers to its distribution that is limited to coastal Western Australia.
Other material examined
Australia: Western Australia: 1 female, Bay of Rest, 22°18'S, 114°08'E (
Diagnosis
See above for Mangrovia albida comb. nov.
Description
Male
(based on holotype,
Female
(based on
Variation
Male only known from holotype; the spine on the median apophysis of the left pedipalp was broken off, therefore the right pedipalp is illustrated here. Female total length 8.7–10.6 (n = 8); there was little colour variation in females although the abdomen venter showed distinct white guanine spots in most specimens.
Habitat preferences and life history
Collection data on labels with museum specimens of M. occidentalis sp. nov. exclusively lists ‘mangroves’ as habitat, where, similar to M. albida comb. nov., spiders were collected mainly from rolled leaves near the orb-web. Mature spiders were mainly collected in May, July and September with a single record in February.
Discussion
Mangrovia gen. nov. males have a single patellar spine on the male pedipalp, a character that was noted by
An association of Mangrovia gen. nov. with the backobourkiines is, however, poorly supported otherwise, as genital morphology of its two species is very unlike other representatives of this clade. The median apophysis of the male pedipalp does not form an arch over the radix as in all other backobourkiines and a basal extension of the conductor, referred to as paramedian apophysis or conductor lobe is absent (e.g.
The pedipalp morphology of Mangrovia gen. nov. is very similar to that of Neoscona, although there are some differences, in particular in the apical section of the pedipalp, including terminal and subterminal apophyses and embolus. The Neoscona pedipalp also has three terminal sclerites, i.e. terminal apophysis, embolus lamella (
As in Neoscona, the radix and stipes are either fused in Mangrovia gen. nov. or a stipes is absent. There seem to be a less sclerotised short section in the radix at least in M. albida comb. nov. (Fig.
Both Mangrovia gen. nov. species appear to be specialists of coastal habitats, particularly inhabiting subtropical mangroves. Here, the apparently nocturnal spiders hide in a self-constructed rolled-leaf retreat adjacent to the web during the day. In Australia, constructing a retreat from a rolled leaf has been observed in other orb-weaving spiders. Apparently similar to Mangrovia gen. nov., Araneus praesignis (L. Koch, 1872) roll a leaf of the plant that harbours the spider’s web (
Acknowledgеments
We acknowledge the support of all museum curators and scientists who facilitated loans of specimens or visits to their respective institutions: Graham Milledge (retired) and Helen Smith (
References
- Berman JD, Levi HW (1971) The orb weaver genus Neoscona in North America (Araneae: Araneidae). Bulletin of the Museum of Comparative Zoology 141: 465–500.
- Coddington JA, Hormiga G, Scharff N (1998) Giant females or dwarf males. Nature 385(6618): 687–688. https://doi.org/10.1038/385687a0
- Court DJ, Forster RR (1988) The spiders of New Zealand: Part VI. Araneidae-Araneinae. Otago Museum Bulletin 6: 68–124.
- Framenau VW (2011) Lariniophora, a new monotypic orb-weaving spider genus from Australia (Araneae: Araneidae: Araneinae). Records of the Western Australian Museum 26(2): 191–201. https://doi.org/10.18195/issn.0312-3162.26(2).2011.191-201
- Framenau VW, Castanheira PdS, Vink CJ (2022) Taxonomy and systematics of the new Australo-Pacifc orb-weaving spider genus Socca (Araneae: Araneidae). New Zealand Journal of Zoology, 1–72. https://doi.org/10.1080/03014223.2021.2014899
- Framenau VW, Dupérré N, Blackledge TA, Vink CJ (2010) Systematics of the new Australasian orb-weaving spider genus Backobourkia (Araneae: Araneidae: Araneinae). Arthropod Systematics & Phylogeny 68: 79–111.
- Framenau VW, Baehr BC, Zborowski P (2014) A guide to the spiders of Australia. New Holland Publishers. London, Chatswood, Auckland, 448 pp.
- Framenau VW, Baptista RLC, Oliveira FSM, Castanheira PdS (2021a) Taxonomic revision of the new spider genus Hortophora, the Australasian Garden Orb-weavers (Araneae, Araneidae). Evolutionary Systematics 5(2): 275–334. https://doi.org/10.3897/evolsyst.5.72474
- Framenau VW, Vink CJ, Scharff N, Baptista RLC, Castanheira P (2021b) Review of the Australian and New Zealand orb-weaving spider genus Novakiella (Araneae, Araneidae). Zoosystematics and Evolution 97(2): 393–405. https://doi.org/10.3897/zse.97.67788
- Grasshoff M (1986) Die Radnetzspinnen-Gattung Neoscona in Afrika. Annales de la Musée Royal de L`Afrique Centrale 250: 1–123.
- Gunnarson B, Johnsson J (1990) Protandry and moulting to maturity in the spider Pityohyphantes phrygianus. Oikos 59(2): 205–212. https://doi.org/10.2307/3545536
- Head G (1995) Selection on fecundity and variaton in the degree of sexual size dimorphism among spider species (class Araneae). Evolution; International Journal of Organic Evolution 49(4): 776–781. https://doi.org/10.1111/j.1558-5646.1995.tb02313.x
- Hormiga G, Scharff N, Coddington JA (2000) The phylogenetic basis of sexual size dimorphism in orb-weaving spiders (Araneae, Orbiculariae). Systematic Biology 49(3): 435–462. https://doi.org/10.1080/10635159950127330
- Joseph MM, Framenau VW (2012) Systematic review of a new orb-weaving spider genus (Araneae: Araneidae), with special reference to the Australasian-Pacific and South-East Asian fauna. Zoological Journal of the Linnean Society 166(2): 279–341. https://doi.org/10.1111/j.1096-3642.2012.00845.x
- Kallal RJ, Hormiga G (2018) Systematics, phylogeny and biogeography of the Australasian leaf-curling orb-weaving spiders (Araneae: Araneidae: Zygiellinae), with a comparative analysis of retreat evolution. Zoological Journal of the Linnean Society 184: 1055–1141. https://doi.org/10.1093/zoolinnean/zly014
- Keyserling E (1887) Die Arachniden Australiens nach der Natur beschrieben und abgebildet. 2. Theil. 3. Lieferung. Bauer & Raspe, Nürnberg, 153–232.
- Koch L (1871) Die Arachniden Australiens nach der Natur beschrieben und abgebildet. 1. Theil. 1. Lieferung. Verlag von Bauer und Raspe, Nürnberg, 1–104. https://doi.org/10.5962/bhl.title.121660
- Kuntner M, Coddington JA (2020) Sexual size dimorphism: evolution and perils of extreme phenotypes in spiders. Annual Reviews of Entomology 65: 57–80. https://doi.org/10.1146/annurev-ento-011019-025032
- Levi H (1993a) American Neoscona and corrections to previous revisions of Neotropical orb-weavers (Araneae: Araneidae). Psyche, Cambridge 99(2–3): 221–239. https://doi.org/10.1155/1992/93912
- Levi H (1993b) The orb-weaver genus Kaira (Araneae: Araneidae). The Journal of Arachnology 21: 209–225.
- Mouginot P, Prügel J, Thom U, Steinhoff POM, Kupryjanowicz J, Uhl G (2015) Securing paternity by mutilating female genitalia in spiders. Current Biology 25(22): 2980–2984. https://doi.org/10.1016/j.cub.2015.09.074
- Moya-Laraño J, Halaj J, Wise DH (2002) Climbing to reach females: Romeo should be small. Evolution; International Journal of Organic Evolution 56(2): 420–425. https://doi.org/10.1111/j.0014-3820.2002.tb01351.x
- Piel WH (1996) Ecology of sexual dimorphism in spiders of the genus Metepeira (Araneae: Araneidae). Review Suisse de Zoologie Volume hors série II: 523–529.
- Rack G (1961) Die Entomologischen Sammlungen des Zoologischen Staatsinstituts und Zoologischen Museums Hamburg. II. Teil. Chelicerata II: Araneae. Mitteilungen des Hamburgischen Zoologischen Museums und Instituts 59: 1–60.
- Rainbow WJ (1911) A census of Australian Araneidae. Records of the Australian Museum 9(2): 107–319. https://doi.org/10.3853/j.0067-1975.9.1911.928
- Rainbow WJ (1916) Arachnida from northern Queensland. Records of the Australian Museum 11: 31–64, 79–119. https://doi.org/10.3853/j.0067-1975.11.1916.912
- Scharff N, Coddington JA (1997) A phylogenetic analysis of the orb-weaving spider family Araneidae (Arachnida, Araneae). Zoological Journal of the Linnean Society 120(4): 355–434. https://doi.org/10.1111/j.1096-3642.1997.tb01281.x
- Scharff N, Coddington JA, Dimitrov D, Agnarsson I, Framenau VW, Szüts T, Blackledge TA (2020) Phylogeny of the orb-weaving spider family Araneidae (Araneae, Araneoidea). Cladistics 36(1): 1–21. https://doi.org/10.1111/cla.12382
- Vollrath F, Parker GA (1992) Sexual dimorphism and distorted sex ratios in spiders. Nature 1992(6400): 156–159. https://doi.org/10.1038/360156a0
- Whyte R, Anderson G (2017) A field guide to the spiders of Australia. CSIRO Publishing, Clayton, Victoria, 451 pp.
- Yin CM, Wang JF, Zhu MS, Xie LP, Peng XJ, Bao YH (1997) Fauna Sinica: Arachnida: Araneae: Araneidae. Science Press, Beijing, 460 pp.
- Yu K-P, Kuntner M, Cheng R-C (2022) Phylogenetic evidence for an independent origin of extreme sexual size dimorphism in a genus of araneid spiders (Araneae: Araneidae). Invertebrate Systematics 36(1): 48–62. https://doi.org/10.1071/IS21019