Research Article |
|
Corresponding author: Baran Yoğurtçuoğlu ( yokbaran@gmail.com ) Academic editor: Nicolas Hubert
© 2021 Mustafa Akkuş, Mustafa Sarı, F. Güler Ekmekçi, Baran Yoğurtçuoğlu.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Akkuş M, Sarı M, Ekmekçi FG, Yoğurtçuoğlu B (2021) The discovery of a microbialite-associated freshwater fish in the world’s largest saline soda lake, Lake Van (Turkey). Zoosystematics and Evolution 97(1): 181-189. https://doi.org/10.3897/zse.97.62120
|
Abstract
Lake Van is the largest saline soda lake in the world and one of the world’s few endorheic lakes of greater than 3,000 km2 surface area. Despite its huge size, no fish species have so far been known to permanently occur in this lake due to its extreme environmental conditions. Here, we report the discovery of a fish population that permanently inhabits some of the unique microbialites of the lake, at a maximum depth of 13 m and about 500 m offshore. We tested whether this is an undescribed species or a new occurrence of a known species. A molecular and morphological examination showed that the newly discovered fish represents an isolated population of Oxynoemacheilus ercisianus, the only nemacheilid loach native to the freshwater tributaries of the Lake Van endorheic basin. Our further hypotheses on the prediction that (a) stream fishes would have a more anterior placement of fins than lake fishes were supported; but, that (b) stream fishes would be more slender bodied than their lake conspecifics was not supported. The lake dwelling population also shows very small sequence divergence (0.5% K2P distance) to its stream dwelling conspecific in the mtDNA-COI barcode region. The notable morphological difference with minute molecular divergence implies that the newly discovered population might have lost its link to freshwater during desiccation and transgressional phases of the Lake Van, and has adapted to a life on the microbialites.
Key Words
Biodiversity, COI, Eastern Anatolia, extreme environments, morphology, Nemacheilidae, Oxynoemacheilus ercisianus
Introduction
Soda lakes are characterized as extreme environments since they are highly alkaline, originate in closed basins, and are often exposed to high evaporation rate (
Nemacheilid loaches are typically found in shallow waters and associated with fast to moderate flowing stretches of the streams, and to a lesser extent, large rivers and lake shores. Habitat associated divergence in morphological traits has been documented in a great number of studies with the best known examples including species from Characidae, Cichlidae (
Material and methods
Fish sampling and measurements
Fish were collected by scuba diving using a small dip net with 4 mm mesh size, by two sampling events carried out in January 2018 and January 2020. After anaesthesia, fish were fixed in 5% formaldehyde and stored in 70% ethanol, or directly stored in 99% ethanol. Measurements were made with a digital calliper and recorded to 0.1 mm. All measurements were made point-to-point, never by projections. Methods for counts and measurements follow
Our hypothesis does not include testing for the variations in body shape. Therefore we used only linear measurements to identify morphological differences between the two groups of fish collected from stream and lake. All body measurements were standardized by individuals’ SL, and the measurements taken from the head region were standardized by individuals’ head length. To reduce the effect of allometric growth on some ratios, we selected similar size range for comparison (i.e., 30.0 mm to 39.2 mm for lake and 30.7 mm to 42.4 mm for stream groups). We also examined univariate patterns to test for significant effect of the explanatory variable as habitat type (stream vs. lake) on each of the response variables (morphometric measurement) using regression model with analysis of covariance (ANCOVA). In this procedure, SL was used as a covariate to control for variation due to fish size (all dependent variables and the covariate were log-transformed) (
The water physicochemical parameters including temperature, pH, dissolved oxygen and salinity were measured at the sampling locations (both in streams and in the lake) using a multiparameter instrument (YSI ProDS, Yellow Spring Instruments, Yellow Springs, OH, USA). The lake measurements were taken during scuba diving, just near the surface of the microbialites where the fish were collected, and not from the open water.
Abbreviations used: SL, standard length; HL, head length. Collection codes: FFR, Recep Tayyip Erdogan University Zoology Museum of the Faculty of Fisheries, Rize, Turkey.
Molecular data analysis
Genomic DNA was extracted from fin tissue using Macherey and Nagel NucleoSpin Tissue kits following the manufacturer’s protocol on an Eppendorf EpMotion® pipetting-roboter with vacuum manifold. The standard vertebrate DNA barcode region of COI was amplified using a M13-tailed primer cocktail including FishF2_t1 (5’TGTAAAACGACGGCCAGTCGACTAATCATAAAGATATCGGCAC), FishR2_t1 5’CAGGAAA- CAGCTATGACACTTCAGGGTGACCGAAGAATCAGAA), VF2_t1 (5’TGTAAAACGACGGCCAGTCAAC- CAACCACAAAGACATTGGCAC) and FR1d_t1 (5’CAGGAAACAGCTATGACACCTCAGGGTGTCC- GAARAAYCARAA) (
Molecular analysis involved 16 nucleotide sequences. Among these, we newly generated 3 DNA barcodes from lake dwelling specimens and included already published data from NCBI GenBank for 11 specimens from Ilıca (Zilan) stream, a northern tributary in Erciş (
List of COI sequences downloaded from NCBI GenBank with information on drainage and country of origin.
Maximum likelihood estimation of the phylogenetic relationships based on the mitochondrial COI barcode region (K2P model, discrete Gamma distribution for rate differences with 3 categories (+G, parameter = 0.0500)). Nucleotide positions with less than 98% site coverage were eliminated, resulting in 570 analysed positions. Numbers of major nodes indicate bootstrap values from 1000 pseudoreplicates from the NJ and ML method.
Results
In total, 44 fish from microbialites and 33 fish from streams were collected and examined. The standard length (SL) of the Oxynoemacheilus individuals from microbialites ranged from 17.8 mm to 39.2 mm, whereas the size of the stream group ranged from 30.7 mm to 66.7 mm.
Molecular and morphological assessments
The analysis of the nucleotide sequences of the COI barcode region resulted in a mean 0.5% K2P distance between the stream and the lake-resident groups, both are separated by 7 variable nucleotide substitutions one of which is unique to the lake group. We treated, therefore, the lake-resident group as conspecific to O. ercisianus.
Despite the low molecular distance, several significant differences were found between the morphological characters of the two populations. The lake-resident population is differentiated from the stream population by the following combination of morphological characters: longer pre-dorsal length (52–56% SL vs. 50–52), longer pre-anal length (78–83% SL vs. 73–77), longer pre-pelvic length (58–61 %SL vs. 52–55) and greater distance between pectoral and pelvic fin origin (33–36% SL vs. 26–31), shorter pelvic fin length (11–13% SL vs. 14–16) and shorter caudal peduncle length (14–16% SL vs 16–18). The pectoral fin length is significantly greater in the stream dwelling population, yet the range overlapped with lake-resident population. Similarly, the snout, post-orbital and inter-orbital distances were significantly greater in the stream population, whereas all overlapped by means of minimum and maximum ratio. See Figs
Nucleotide substitutions in the variable sites of the mitochondrial COI gene (570 bp) of Oxynoemacheilus ercisianus from lake and stream populations.
| Nucleotide position | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 3 | 4 | 4 | ||||
| 3 | 4 | 3 | 8 | 8 | 8 | ||
| Individuals | 8 | 3 | 5 | 1 | 0 | 2 | 5 |
| Oxynoemacheilus ercisianus MW684715 (Lake) | T | C | C | C | T | G | A |
| Oxynoemacheilus ercisianus MW684714 (Lake) | . | . | . | T | . | . | . |
| Oxynoemacheilus ercisianus MW684713 (Lake) | . | . | . | . | . | . | . |
| Oxynoemacheilus ercisianus KU928283 (Stream) | C | . | . | . | . | . | G |
| Oxynoemacheilus ercisianus MK546487 (Stream) | . | A | A | . | . | . | . |
| Oxynoemacheilus ercisianus MH469267 (Stream) | . | . | A | . | C | . | G |
| Oxynoemacheilus ercisianus MH469268 (Stream) | . | . | A | . | . | . | G |
| Oxynoemacheilus ercisianus MK546488 (Stream) | . | A | A | . | . | . | . |
| Oxynoemacheilus ercisianus MK546486 (Stream) | . | A | A | . | . | A | . |
| Oxynoemacheilus ercisianus MK546485 (Stream) | . | . | A | . | . | . | G |
| Oxynoemacheilus ercisianus MK546484 (Stream) | . | . | A | . | C | . | G |
| Oxynoemacheilus ercisianus MK546454 (Stream) | C | . | . | . | . | . | G |
| Oxynoemacheilus ercisianus MK546453 (Stream) | C | . | . | . | . | . | G |
| Oxynoemacheilus ercisianus MK546452 (Stream) | C | . | . | . | . | . | G |
The lake-resident population is further differentiated from the stream population by having a shorter lateral line with 5–7 pores reaching up to vertical of pectoral fin midline (vs. 9–12 pores reaching up to pectoral fin tip or dorsal fin origin). Usually, there are none, or just one faint lateral pore in supratemporal canal (vs. two or three apparent pores). All other meristic traits including fin ray numbers overlapped between lake and stream dwelling populations.
The SL of the lake-resident population ranged from 17.8 mm to 39.2 mm, suggesting smaller maximum size compared to the stream population (ranged from 30.7 mm to 66.7 mm SL).
Material examined
Oxynoemacheilus ercisianus (stream dwelling population): FFR 15533, 3, 35–48 mm SL, Turkey: Van prov.: Ilıca stream at Erciş, 2 km northwest to Ulupamir, 39.1813, 43.3019. –FFR 15534, 3, 38–49 mm SL; Turkey: Van prov.: Ilıca stream at Erciş Örene, under the bridge at Bitlis-Tatvan Road, 39.0063, 43.3180. –Uncat., 27, 31–67 mm SL, Turkey: Van prov.: Ilıca stream 20 km north of Erciş, 39.2264, 43.3887.
Oxynoemacheilus ercisianus (lake-resident population)–Uncat., 19, 18–36 mm SL, Turkey: Van prov.: gulf near Gevaş in the southern Lake Van, 38.3168, 43.1149. –FFR 01403, 25, 19–39 mm SL, Turkey: Van prov.: shore of Edremit, Lake Van, 38.4250, 43.2319.
Material used in molecular genetic analysis
Habitat characteristics
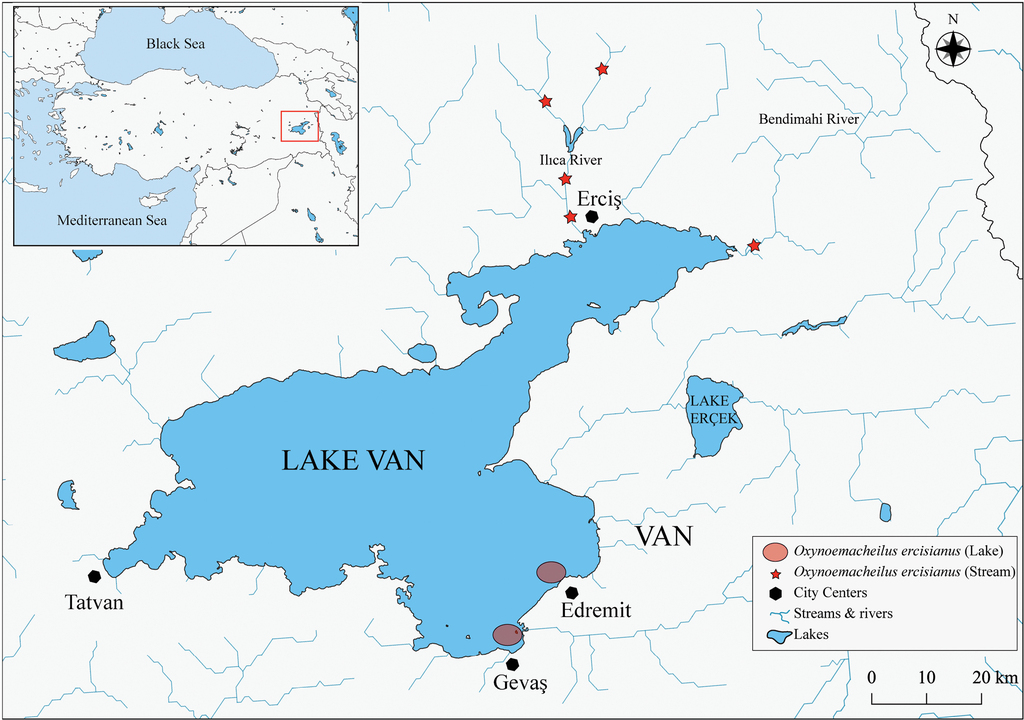
The lake-resident populations of O. ercisianus were found from two microbialite areas on the south-eastern coast of the Lake Van (Fig.
Morphometric data of Oxynoemacheilus ercisianus from lake and stream populations (Lake, FFR 01403, n = 12; Stream, FFR 15533, n = 10). Bold text – both significant at p < 0.01 (ANCOVA) and non-overlapping ranges.
| Lake-resident population | Stream population | Significance | |||||||
|---|---|---|---|---|---|---|---|---|---|
| mean | min | max | SD | mean | min | max | SD | p-Value | |
| Standard length (mm) | 30 | 39 | 31 | 42 | |||||
| In percent of standard length | |||||||||
| Head length | 24.7 | 23.3 | 26.4 | 1.0 | 25.7 | 24.0 | 27.6 | 1.0 | 0.020 |
| Body depth at dorsal–fin origin | 15.9 | 14.0 | 17.3 | 1.3 | 17.2 | 16.3 | 20.1 | 1.2 | 0.266 |
| Pre-dorsal length | 53.8 | 52.1 | 56.0 | 1.3 | 50.8 | 49.8 | 51.7 | 0.8 | < 0.001 |
| Pre-pelvic length | 59.2 | 58.2 | 60.7 | 0.8 | 53.2 | 51.9 | 54.7 | 1.0 | < 0.001 |
| Pre-pectoral length | 28.9 | 28.4 | 29.6 | 0.4 | 28.3 | 27.1 | 29.5 | 0.9 | 0.233 |
| Pre-anal length | 80.0 | 77.8 | 82.8 | 1.5 | 74.9 | 73.1 | 77.0 | 1.2 | < 0.001 |
| Post-dorsal length | 35.1 | 33.8 | 37.9 | 1.0 | 37.0 | 35.0 | 38.6 | 1.3 | 0.003 |
| Distance between pec. and pel-fin origins | 34.6 | 33.0 | 35.7 | 1.0 | 29.2 | 26.4 | 30.6 | 1.3 | < 0.001 |
| Distance between vent and anal-fin origin | 2.9 | 2.3 | 3.3 | 0.3 | 3.2 | 2.8 | 3.7 | 0.3 | 0.490 |
| Distance between pel. and anal-fin origins | 21.2 | 19.2 | 21.9 | 0.8 | 21.4 | 20.1 | 22.1 | 0.7 | 0.638 |
| Dorsal-fin length | 17.5 | 16.2 | 19.5 | 1.0 | 20.2 | 19.1 | 21.7 | 0.9 | < 0.001 |
| Anal-fin base length | 7.5 | 6.7 | 9.0 | 0.6 | 8.2 | 6.9 | 9.0 | 0.6 | 0.012 |
| Pectoral-fin length | 17.6 | 16.0 | 19.7 | 1.3 | 20.2 | 18.2 | 22.0 | 1.4 | 0.006 |
| Pelvic-fin length | 12.2 | 11.2 | 13.3 | 0.7 | 14.8 | 13.6 | 15.9 | 0.8 | < 0.001 |
| Length of caudal peduncle | 14.5 | 13.5 | 16.1 | 0.8 | 17.0 | 16.2 | 18.2 | 0.6 | < 0.001 |
| Depth of caudal peduncle | 9.1 | 8.2 | 10.1 | 0.7 | 10.2 | 9.6 | 10.8 | 0.5 | 0.012 |
| In percent of head length | |||||||||
| Snout length | 35.9 | 34.4 | 37.5 | 1.0 | 38.0 | 36.3 | 40.0 | 1.5 | 0.003 |
| Eye diameter | 22.1 | 19.9 | 25.0 | 1.3 | 20.4 | 18.6 | 22.6 | 1.4 | 0.565 |
| Interorbital width | 33.2 | 30.6 | 35.1 | 1.4 | 35.3 | 32.7 | 37.5 | 1.8 | < 0.001 |
| Postorbital distance | 45.0 | 42.3 | 47.4 | 1.7 | 47.2 | 45.7 | 48.6 | 1.1 | 0.001 |
| Maximum head width | 63.4 | 61.8 | 65.4 | 1.2 | 64.6 | 61.8 | 70.2 | 2.8 | 0.065 |
| Head depth at eye | 45.9 | 43.8 | 48.0 | 1.2 | 49.0 | 45.2 | 51.7 | 2.4 | 0.005 |
| Length of inner rostral barbel | 17.1 | 16.3 | 19.8 | 1.0 | 16.4 | 14.4 | 18.2 | 1.3 | 0.832 |
| Length of outer rostral barbel | 19.2 | 17.3 | 21.2 | 1.3 | 17.8 | 16.3 | 19.6 | 1.2 | 0.363 |
| Length of maxillary barbel | 22.2 | 20.7 | 23.6 | 1.0 | 20.1 | 18.4 | 22.7 | 1.4 | 0.070 |
Water physico-chemical parameters measured in two microbialite areas (in Edremit and Gevaş and in Bendimahi river.
| Parameter | Edremit (Lake) | Gevaş (Lake) | Bendimahi (Stream) |
|---|---|---|---|
| Water temperature (C°) | 11 | 12.3 | 6.8 |
| Salinity (‰) | 18.1 | 18.3 | 0.4 |
| pH | 9.1 | 9.2 | 7.9 |
| Dissolved Oxygen (mg/L) | 7.6 | 7.9 | 10.2 |
| Total Dissolved Solids (g/L) | 19.3 | 20.1 | 15.6 |
Discussion
The findings of the present study have entirely changed the generally accepted knowledge that no fish species is found to permanently occur in Lake Van. Our discovery of the first fish, a nemacheilid loach, permanently inhabiting the lake, triggered the question whether it might be a new or undescribed species. Our hypothesis testing resulted in recognizing the fish as a distinct and isolated population of Oxynoemacheilus ercisianus, the only nemacheilid species in the endorheic Lake Van basin. Despite considerable differences in some of the morphometric characters between the newly found lake-resident population and its stream conspecific, the two groups are superficially very similar to each other with also very small K2P distance in their COI barcode region. We follow
To answer how O. ercisianus might have been locked in the microbialites of the Lake Van is not easy. However, according to the geological support, the Lake Van had been exposed to a combination of rapid desiccation and transgressional phases throughout the Holocene and late Pleistocene (
Acknowledgements
We would like to thank Hayrullah Söylemez and Ali Haydar Kapkaç, the members of the Underwater Team, and the members of the Public Security Boat Team of the Van Gendarmerie Command, for their help in diving for fish sampling. Many thanks to Saygun Dura, who photographed the fish in its habitat and allowed us to use these photographs. We would like to thank Jörg Freyhof (Berlin) and Matthias Geiger (Bonn) for sharing the mtDNA COI sequences of O. ercisianus from the lake habitat with us. We would like to thank Harun Aydın (Ankara) for his help in understanding the microbialite hydrology.
References
- Brinsmead J, Fox MG (2002) Morphological variation between lake‐ and stream‐dwelling rock bass and pumpkinseed populations. Journal of Fish Biology 61(6): 1619–1638. https://doi.org/10.1111/j.1095-8649.2002.tb02502.x
- Costa-Pereira R, Araújo MS, Paiva F, Tavares LER (2016) Functional morphology of the tetra fish Astyanax lacustris differs between divergent habitats in the Pantanal wetlands. Journal of Fish Biology 89(2): 1450–1458. https://doi.org/10.1111/jfb.13026
- Degens ET, Wong HT, Kempe S, Kurtman FA (1984) Geological Study of Lake Van, Eastern Turkey. Geol Rundsch 73(2): 701–734. https://doi.org/10.1007/BF01824978
- Dunn NR, O’Brien LK, Burridge CP, Closs GP (2020) Morphological convergence and divergence in Galaxias fishes in Lentic and Lotic habitats. Diversity 12(183): 1–25. https://doi.org/10.3390/d12050183
- Edgar RC (2004) MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic acids research 32: 1792–1797. https://doi.org/10.1093/nar/gkh340
- Elp M, Atıcı AA, Şen F, Duyar HA (2016) Distribution of fish species in the Van Lake basin. Yüzüncü Yıl Üniversitesi Tarım Bilimleri Dergisi 26(4): 563–568. https://doi.org/10.29133/yyutbd.282808
- Franssen NR, Stewart LK, Schaefer JF (2013) Morphological divergence and flow‐induced phenotypic plasticity in a native fish from anthropogenically altered stream habitats. Ecology and Evolution 3(14): 4648–4657. https://doi.org/10.1002/ece3.842
- Freyhof J, Geiger MF, Golzarianpour K, Patimar R (2016) Sasanidus, a new generic name for Noemacheilus kermanshahensis Bănărescu & Nalbant, with discussion of Ilamnemacheilus and Schistura (Teleostei; Nemacheilidae). Zootaxa 4107(1): 65–80. https://doi.org/10.11646/zootaxa.4107.1.3
- Freyhof J, Bayçelebi E, Geiger MF (2018) Review of the genus Cobitis in the Middle East, with the description of eight new species (Teleostei: Cobitidae). Zootaxa: 4535(1): 1–75. https://doi.org/10.11646/zootaxa.4535.1.1
- Geiger MF, Herder F, Monaghan MT, Almada V, Barbieri R, Bariche M, Berrebi P, Bohlen J, Casal-Lopez M, Delmastro GB, Denys GPJ, Dettai A, Doadrio I, Kalogianni E, Kärst H, Kottelat M, Kovačić M, Laporte M, Lorenzoni M, Marčić Z, Özuluğ M, Perdices A, Perea S, Persat H, Porcelotti S, Puzzi C, Robalo J, Šanda R, Schneider M, Šlechtová V, Stoumboudi M, Walter S, Freyhof J (2014) Spatial heterogeneity in the Mediterranean Biodiversity Hotspot affects barcoding accuracy of its freshwater fishes. Molecular Ecology Resources 14: 1210–1221. https://doi.org/10.1111/1755-0998.12257
- Haas TC, Blum MJ, Heins DC (2010) Morphological responses of a stream fish to water impoundment. Biology Letters 6(6): 803–806. https://doi.org/10.1098/rsbl.2010.0401
- Ivanova NV, Zemlak TS, Hanner RH, Hebert PDN (2007) Universal primer cocktails for fish DNA barcoding. Molecular Ecology Notes 7: 544–548. https://doi.org/10.1111/j.1471-8286.2007.01748.x
- Jones BE, Grant WD, Duckworth AW, Owenson GG (1998) Microbial diversity of soda lakes. Extremophiles 2(3): 191–200. https://doi.org/10.1007/s007920050060
- Kaya C (2020) The first record and origin of Salmo trutta populations established in the Upper Tigris River and Lake Van Basin (Teleostei: Salmonidae). Journal of Anatolian Environmental and Animal Science 5(3): 366–372. https://doi.org/10.35229/jaes.777575
- Kempe S, Kazmierczak J (2011) Soda Lakes. In: Reitner J, Thiel V (Eds) Encyclopedia of Geobiology. Encyclopedia of Earth Sciences Series. Springer, Dordrecht, 956 pp. https://doi.org/10.1007/978-1-4020-9212-1_191
- Kempe S, Kazmierczak J, Landmann G, Konuk T, Reimer A, Lipp A (1991) Largest known microbialites discovered in Lake Van, Turkey. Nature 349(6310): 605–608. https://doi.org/10.1038/349605a0
- Kottelat M, Freyhof J (2007) Handbook of European Freshwater Fishes. Kottelat, Cornol and Freyhof, Berlin, 646 pp.
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Molecular Biology and Evolution 35(6): 1547–1549. https://doi.org/10.1093/molbev/msy096
- Langerhans RB (2008) Predictability of phenotypic differentiation across flow regimes in fishes. Integrative and Comparative Biology 48(6): 750–768. https://doi.org/10.1093/icb/icn092
- Langerhans RB, Layman CA, Langerhans AK, DeWitt TJ (2003) Habitat-associated morphological divergence in two Neotropical fish species. Biological Journal of the Linnean Society 80(4): 689–698. https://doi.org/10.1111/j.1095-8312.2003.00266.x
- McGuigan K, Franklin CE, Moritz C, Blows MW (2003) Adaptation of rainbow fish to lake and stream habitats. Evolution 57: 104–118. https://doi.org/10.1111/j.0014-3820.2003.tb00219.x
- McLaughlin RL, Noakes DLG (1998) Going against the flow: an examinationof the propulsive movements made by young brook trout in streams. Canadian Journal of Fisheries and Aquatic Sciences 55: 853–860. https://doi.org/10.1139/f97-308
- Perazzo GX, Corrêa F, Salzburger W, Gava A (2019) Morphological differences between an artificial lentic and adjacent lotic environments in a characid species. Reviews in Fish Biology and Fisheries 29: 935–949. https://doi.org/10.1007/s11160-019-09582-y
- Pinti DL (2014) Soda Lakes. In: Gargaud M, Irvine WM, Amils R, Cleaves HJ, Pinti D, Quintanilla JC, Viso M (Eds) Encyclopedia of Astrobiology. Springer, Berlin, Heidelberg, 1853 pp. https://doi.org/10.1007/978-3-642-27833-4_1455-6
- Reimer A, Landmann G, Kempe S (2009) Lake Van, Eastern Anatolia, Hydrochemistry and History. Aquatic Geochemistry 15: 195–222. https://doi.org/10.1007/s10498-008-9049-9
- Sarı M (2008) Threatened fishes of the world: Chalcalburnus tarichi (Pallas 1811) (Cyprinidae) living in the highly alkaline Lake Van, Turkey. Environmental Biology of Fishes 81: 21–23. https://doi.org/10.1007/s10641-006-9154-9
- Schagerl M, Burian A (2016) The Ecology of African Soda Lakes: Driven by Variable and Extreme Conditions. In: Schagerl M (Ed.) Soda Lakes of East Africa. Springer, Cham, 295–320. https://doi.org/10.1007/978-3-319-28622-8_12
- Swain DP, Holtby LB (1989) Differences in morphology and behaviour between juvenile coho salmon (Oncorhynchus kisutch) rearing in a lake and in its tributary stream. Canadian Journal of Fisheries and Aquatic Sciences 46: 1406–1414. https://doi.org/10.1139/f89-180
- Turan D, Kaya C, Kalayci G, Bayçelebi E, Aksu İ (2019) Oxynoemacheilus cemali, a new species of stone loach (Teleostei: Nemacheilidae) from the Çoruh River drainage, Turkey. Journal of Fish Biology 94(3): 458–468. https://doi.org/10.1111/jfb.13909
- Webb PW (1984) Body form, locomotion, and foraging in aquatic vertebrates. American Zoologist 24: 107–120. https://doi.org/10.1093/icb/24.1.107
- Yoğurtçuoğlu B, Kaya C, Geiger MF, Freyhof J (2020) Revision of the genus Seminemacheilus, with the description of three new species (Teleostei: Nemacheilidae). Zootaxa 4802(3): 477–501. https://doi.org/10.11646/zootaxa.4802.3.5
- Zar JH (2010) ‘Biostatistical Analysis’. Prentice Hall International: Upper Saddle River, NJ, USA.