Research Article |
|
Corresponding author: Azman Abdul Rahim ( abarahim@gmail.com ) Academic editor: Matthias Glaubrecht
© 2015 Azman Abdul Rahim, Ali Eimran Alip.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Abdul Rahim A, Eimran Alip A (2015) First record of Parelasmopus setiger Chevreux, 1901 from Singapore, including synonymization of Parelasmopus siamensis Wongkamhaeng, Coleman & Pholpunthin, 2013 with Parelasmopus setiger (Crustacea, Amphipoda, Maeridae). Zoosystematics and Evolution 91(1): 59-70. https://doi.org/10.3897/zse.91.9014
|
Abstract
Parelasmopus setiger has been widely described from tropical to subtropical regions from Philippine Islands, Sulu Sea, Indonesia, Australia, north Indian Ocean and the Seychelles by several authors. The present study provides detailed descriptions of Parelasmopus setiger Chevreux, 1901 (Crustacea: Amphipoda) based on newly collected specimens from Pulau Hantu, Singapore. Morphological characters of the specimens closely resemble those of Parelasmopus setiger and Parelasmopus siamensis Wongkamhaeng, Coleman & Pholpunthin, 2013. The specimens of both the species were quite similar to the Singapore specimens, although the shapes of appendages vary with growth and locality; therefore, P. siamensis is synonymized with P. setiger. The following species characteristics for P. setiger are indicated: 1) antenna 1 peduncle with 2 setae; 2) male gnathopod 2 propodus palm transverse; 3) Pereopods 5 to 7 posterior margins with long slender setae; and 4) dorsal carina pattern for pereonite 7 and pleonites 1 to 3.
Key Words
Amphipoda , Maeridae , Singapore, Parelasmopus setiger , Parelasmopus siamensis , synonym
Introduction
Few works have been published on amphipods from Singapore and most are a century old. These works include
Materials and methods
This study was based on material collected in July 2013, from the shallow-water coral reef habitats of Pulau Hantu, Singapore (Fig.
Systematics
Suborder Senticauda Lowry & Myers, 2013
Maeridae Krapp-Schickel, 2008
Parelasmopus
Type species
Megamoera suensis (Haswell, 1879).
Diagnosis
Head with notch on cheek. Mandible mandibular palp 3-articulate, article 2 much shorter than article 1, article 3 straight, not setiferopectinate. Urosomite 1 with pair of dorsal carinae. Uropod 3 rami length subequal to peduncle; Epimeron 3 posteriorly serrate on lower margin. (After
Species composition
Parelasmopus includes 13 species: P. albidus (Dana, 1853); P. aumogo Hughes, 2011; P. cymatilis Lowry & Hughes, 2009; P. echo J.L. Barnard, 1972a; P. dancaui Ortiz & Lalana, 1997; P. mallacootaformis Ledoyer, 1984; P. poorei Hughes 2009; P. setiger Chevreux, 1901; P. sowpigensis Lowry & Springthorpe, 2005; P. suensis (Haswell, 1879); P. suluensis (Dana, 1852); P. ya J.L. Barnard, 1972 and P. zelei Ledoyer, 1983.
Parelasmopus setiger
? Megamoera suensis Haswell, 1880c: 335–336, pl. 21: fig. 5.
Megamoera suensis. – Miers 1884: 317–318.
Megamoera haswelli Miers, 1884: 318 [name in text].
Not Megamoera suensis. – Haswell 1885: 103–104, pl. 15: figs 1–4 [=Maera hamigera (Haswell) fide Stebbing, 1906, but see Stebbing, 1910a: 600].
Elasmopus suensis. – Stebbing 1906: 442–443.
Parelasmopus setiger Chevreaux, 1901: 412–418, fig. 32–39.
Parelasmopus suluensis. – Chilton 1922: 7–8, fig. 3 [not Dana].
? Parelasmopus suluensis. – Walker 1904: 278, pl. 6: fig. 3 [?not Dana]
Parelasmopus siamensis Wongkamhaeng, Coleman & Pholpunthin, 2013: 525–532, figs 19–24.
Material examined
– 1 male, 10.3 mm, UKMMZ-1527, shallow water coral habitat of Pulau Hantu, Singapore, 1°13’37.9”N, 103°44’27.6”E, mesh bath netting, 5 m, coll. E.A. Ali, Tan, Y.K. and Lee, A.C., 26 November 2013: 3 males; 1 female; 2 juveniles, UKMMZ-1529, same station data.
Type locality
Port Of Victoria, Mahé, Seychelles.
Description
Based on male, 10.3 mm, UKMMZ-1527.
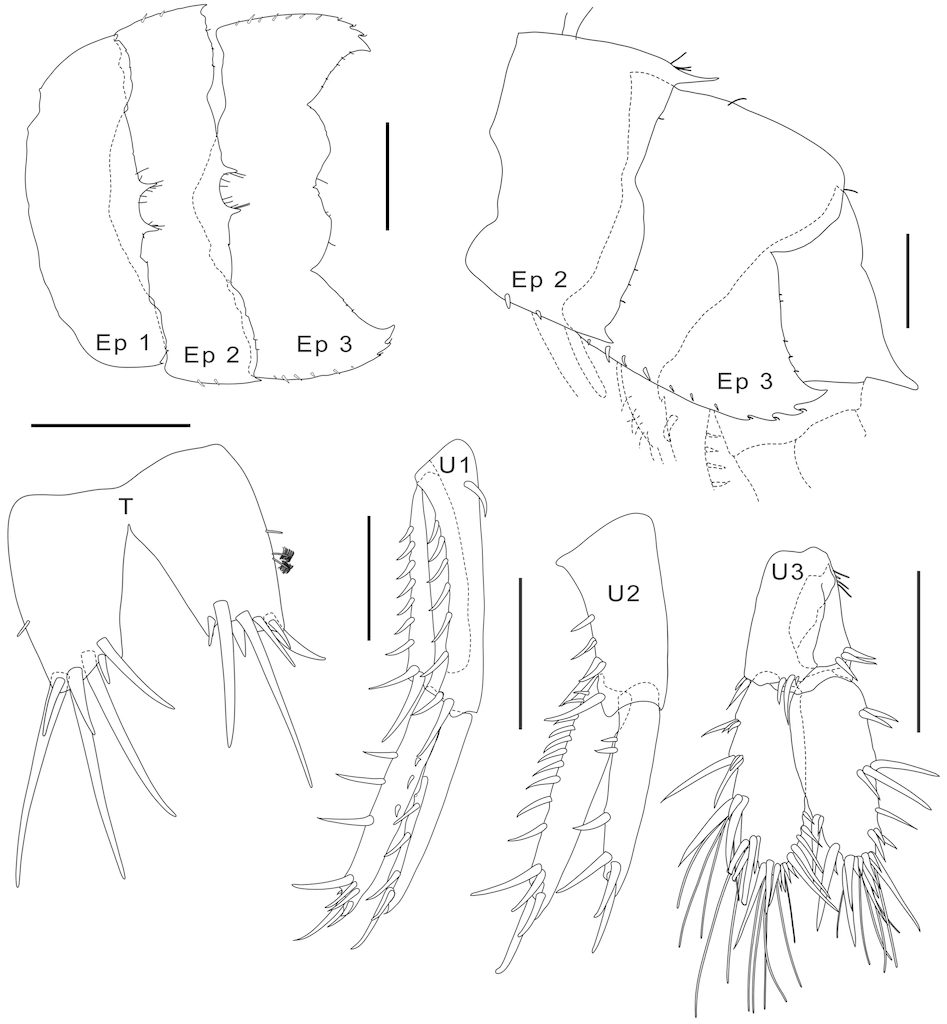
Head.Head slightly longer than pereonites 1–2 combined; rostrum small; lateral cephalic lobe broad, moderately produced, anteroventral margin with notch/slit; eyes well developed, ovate. Urosomite 1–3 serrated dorsally. Antenna 1 longer than antenna 2; peduncular article 1 subequal to article 2, with 2 robust setae along posterior margin, ventrodistal margin with 1 group of robust setae and 2 fine setae; article 2 with several fine setae along both margins; flagellum articles broader than long, with 18+ articles (broken); accessory flagellum minute, with 3 plus one rudimentary article. Antenna 2 peduncular article 2 cone gland reaching beyond peduncular article 3; article 4 longer than article 5; flagellum with 11 articles. Upper lip semicircular, pubescent. Lower lip outer lobes with two pairs of ducts, mandibular lobes apically subacute. Maxilla 1 inner plate subtriangular, with 2 apical plumose setae; outer plate armed with 6 serrate robust setae, facial side with row of 5 serrate robust setae; palp biarticulate, article 1 shorter than article 2, article 2 with 6 thick setae terminally. Maxilla 2 outer plate slightly broader than inner plate; inner plate armed with 13 mostly long setae from distal end to inner half margin; outer plate with 10 setae only on apex. Mandible (left), incisor armed with 4 teeth; lacinia mobilis serrate apically; accessory setal row well developed and composed of 4 setae and 1 broad plate; molar process well developed, triturative, provided with 2 plumose setae and 1 stout seta; mandibular condyle well developed; palp triarticulate, article 1 distally swollen, article 2 short almost 2.5 times as long as article 3, article 3 and apically provided with 1 couple of long setae.
Pereon.Gnathopod 1 smaller than gnathopod 2; coxa 1 anterior margin concave, produced forward anteroventrally, posteroventral corner notched; basis anterior margin straight with 3 short robust setae along the margin, posterodistally provided with several plumose setae, posterior margin with several long setae on mid length; ischium subcyclindrical, about 33% as long as basis, posterodistally provided by 3 plumose and 2 medium length setae; merus slightly longer than ischium with a row of short and long setae on distal half; carpus as long as propodus, anterodistally with 3 long and 2 short setae, medial surface with rows of pectinate setae; propodus medial surface with comb, palm convex defined by 1 pronounced spine, marginally provided with about 7 bifid small spines and many minute setae; dactylus falcate, overlapping palm.
Gnathopod 2 coxa 2 subquadrate, posteroventral corner notched; basis slender with slight excavation along anterior margin, anterodistal corner subquadrate, posterior margin with medium length setae on mid length; merus acutely produced distoventrally; carpus compressed, subtriangular, posterior expansion densely setaceous in rows, anterodistally with 2 stout setae; propodus expanded and subrectangular, posterior margin with thick setae, palm transverse, defined by 9 robust setae along the palmar margin, deeply concave medially; dactylus falcate, with crenulated posteroproximal shelf.
Pereopods 3–4 coxa 3 produced forward on ventral half part of anterior margin. coxa 4 broader than deep, posteroventral lobe well developed, with subrectangular posteromedial corner. Pereopods 3–4 similar except for pereopod 3 shorter; carpus posterodistally provided with 2 or more prominent robust setae; propodus posteriorly provided with row of short robust setae. Pereopods 5–7 coxae concave, both anterodistally and posterodistally, basis slightly expanded, posterior margin crenulate, without long slender setae, except for pereopod 7; merus and carpus not broadened; carpus and propodus with, slender setae along posterior margin; propodus not expanded posterodistally; dactylar ungues simple.
Urosome.Urosomite 1–3 dorsally bicarinate. Epimeron 1–3 posteroventral corner with small acute spine. Epimeron 3 ventral margin serrate distally, posteroventral margin serrate below posteroventral corner, posteroventral corner with strongly produced acute spine. Uropod 1 extending beyond peduncle of uropod 3; peduncle longer than rami, outer-ventrodistally provided with 1 robust seta, upper margin with 8 inner and 9 outer robust setae; rami subequal, truncate, apically provided with 1 pronounced and 2 robust setae, outer ramus with 3 outer robust setae, inner ramus with 4 outer small setae and 3 inner median robust setae. Uropod 2 not extending beyond peduncle of uropod 3; peduncle shorter than rami, upper-marginally provided with 5 median robust setae, 1 inner-distal robust seta; rami subequal, truncate, outer ramus provided with 4 median robust setae, 1 distal robust seta and 1 apical robust seta, inner ramus provided with 10 inner median and 3 inner distal robust seta, and 1 apical robust setae. Uropod 3 peduncle 33% as long as outer ramus, provided with 2 outer, 3 inner and 2 distal robust setae; rami foliaceous, both rami distally truncated to subacute, with long and short apical robust setae. Telson broader than long, small, 5/6 cleft, each lobe with slight ridges on central line, with 7 distal robust setae.
Female (dimorphic characters). Based on female, 9.8 mm, UKMMZ-1528.
Gnathopod 2 carpus relatively long about, 1.5 times as long as wide, slightly lobate; propodus linear, almost five times as long as broad, without distomedial shelf; dactylus apically subacute.
Remarks
Both
The recently described P. siamensis
The present comparison suggests that further taxonomic studies on this species group are necessary. Detailed drawings and descriptions provided in this study could aid in eliminating further confusion within the P. setiger complex, including and thus establish its definitive characteristics.
Distribution
Seychelles, Philippine Islands, Sulu Sea, Indonesia, Gulf of Thailand, Australia, north Indian Ocean and Singapore (current study).
Acknowledgments
We gratefully acknowledge the generous assistance of Dr. Tan Koh Siang (Head of Marine Biology and Ecology Lab) during the first author visit to the Tropical Marine Science Institute (TMSI), Singapore. Thanks are also due to Dr. Sin Tsai Min (Head of Ecological Monitoring, Informatics and Dynamics Lab; Senior Research Fellow for the Coastal Marine Cluster) for her continuous support and encouragement. ABAR was partially supported by the Universiti Kebangsaan Malaysia research grant (AP-2013-005 and FRGS/1/2014/STWN10/UKM/02/6) for his short visit to the TMSI.
Reference
- Barnard JL (1972) Gammaridean Amphipoda of Australia. Part 1. Smithsonian Contributions to Zoology 103: 1–333.
- Chevreux E (1901) Crustacés Amphipodes. In: Mission scientifique de M. Ch. Alluaud aux Iles Séchelles (Mars, Avril, Mai 1892). Mémoires de la Société zoologique de France 14: 388–438.
- Coleman CO (2003) “Digital inking”: How to make perfect line drawings on computers. Organisms, Diversity and Evolution 3(14): 1–14. doi: 10.1078/1439-6092-00081
- Dana JD (1852) On the classification of the CrustaceaChoristopoda or Tetradecapoda. American Journal of Science and Arts, Series 2, 14: 297–316.
- Dana JD (1853) Crustacea. Part IS. United States Exploring Expedition 14: 689–1618.
- Haswell WA (1879) On some additional new genera and species of amphipodous crustaceans. Proceedings of the Linnean Society of New South Wales 4: 319–350.
- Hughes LE (2011) New species of Hoho, Mallacoota and Parelasmopus (Maeridae: Amphipoda) from Australian waters. Zootaxa 2955: 1–79.
- Krapp-Schickel T (2008) What has happened with the Maera-clade (Crustacea, Amphipoda) during the last decades? Bollettino del Museo Civico di Storia Naturale di Verona, Botanica Zoologia 32: 3–32.
- Ledoyer M (1983) Crustacés amphipodes gammariens. Familles des Acanthonotozomatidae à Gammaridae. Faune de Madagascar 59(1): 1–598.
- Ledoyer M (1984) Les gammariens (Crustacea, Amphipoda) des herbiers de phanérogames marines de Nouvelle Calédonie (région de Nouméa). Mémoires du Muséum National d’Histoire Naturelle, Series A, Zoology 129: 1–113.
- Lowry JK, Hughes LE (2009) Maeridae, the Elasmopus group. In: Lowry JK, Myers AA (Eds) Amphipoda of the Great Barrier Reef, Australia.Zootaxa 2260: 643–702.
- Lowry JK, Myers AA (2013) A Phylogeny and Classification of the Senticaudata subord. nov. (Crustacea: Amphipoda). Zootaxa 3610(1): 1–80. doi: 10.11646/zootaxa.3610.1.1
- Lowry JK, Springthorpe RT (2005) New and little-known melitid amphipods from Australian waters (Crustacea: Amphipoda: Melitidae). Records of the Australian Museum 57: 237–302. doi: 10.3853/j.0067-1975.57.2005.1463
- Mayer P (1903) Die Caprelliden der Siboga-Expedition. Siboga-Expeditie, Monographie 34: 1–160.
- Ortiz M, Lalana R (1997) Amphipoda. In: Gutu M (Ed.) Results of the Zoological Expedition Organized by “Grigore Antipa” Museum in the Indonesian Archipelago (1991). 1. Percarida (Crustacea). Travaux du Muséum National d’Histoire Naturelle “Grigore Antipa”, 29–113.
- Stebbing TRR (1887) On some new exotic Amphipoda from Singapore and New Zealand. Transactions of the Royal Society of London 12(6): 199–209, pls 38–39. doi: 10.1111/j.1096-3642.1887.tb00013.x
- Stebbing TRR (1888) Report on the Amphipoda collected by H.M.S. Challenger during the years 1873–1876. Report on the Scientific Results of the Voyage of H.M.S. Challenger during the years 1873–76. Zoology 29: 1–1737.
- Tattersall WM (1922) Amphipoda and Isopoda. The Percy Sladen Trust Expeditions to the Abrolhos Islands (Indian Ocean). Journal of the Linnean Society of London, Zoology 35: 1–19. doi: 10.1111/j.1096-3642.1922.tb01493.x
- Watling L (1989) A classification system for crustacean setae based on the homology concept. In: Felgenhauer BE, Watling L, Thistle AB (Eds) Functional Morphology of Feeding and Grooming in Crustacea. Crustacean Issue 6, Balkema, Rotterdam, 15–27.
- Wongkamhaeng K, Coleman CO, Pholpunthin P (2013) Three new species from the Aoridae and Maeridae (Crustacea, Amphipoda) from Thai waters. Zootaxa 3693: 503–533. doi: 10.11646/zootaxa.3693.4.6