Research Article |
|
Corresponding author: Peter Bartsch ( peter.bartsch@mfn-berlin.de ) Academic editor: Matthias Glaubrecht
© 2017 Honesty Yanwirsal, Peter Bartsch, Frank Kirschbaum.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Yanwirsal H, Bartsch P, Kirschbaum F (2017) Reproduction and development of the asian bronze featherback Notopterus notopterus (Pallas, 1769) (Osteoglossiformes, Notopteridae) in captivity. Zoosystematics and Evolution 93(2): 299-324. https://doi.org/10.3897/zse.93.13341
|
Abstract
Experimental data demonstrate that the environmental factors decreasing conductivity, slight variation of temperature, and water level have no influence on gonad development or courtship behavior in Notopterus notopterus. Spawning occurs during day time at a temperature of 25–28 °C. Newly spawned 3.8–4 mm adhesive eggs are guarded by the male until hatching. The egg envelope has external spiraling ridges, which are centered round the micropyle. Hatching occurs within 168–204 hours depending on temperature and even with some variability at 27°C constant incubation temperature. Exogenous feeding then starts on day 17 with a total length of 16.2 mm and yolk-sac remnants still present. The larval period lasts until day 36. Dark brown stripes appear on the body as one of the characteristic pigment patterns of juvenile N. notopterus at day 70 with a total length of around 34 mm, replacing the dotted pigment pattern of larvae and early juveniles. Later again a spectacular color change to uniformly gold-bronze coloration occurs. The genital papilla can macroscopically be recognized at day 80. Sexual maturity of N. notopterus in captivity as indicated by courtship behavior is first observed in 30-month old specimens of both sexes of the F1 generation with a total length of around 275 mm in males and 230 mm in females, whereas this might occur at smaller size in the P generation and in natural environment. Generally, in N. notopterus the embryonic period lasts longer and the onset of the larval period starts much delayed as compared to a typical indirect or saltatory development. The larval period before onset of the juvenile period with its spectacular color changes, shows few discernible stages of morphological development. It is immediately a pterygiolarva with the jaws, branchial arches, most fins differentiated, a distinct pigmentation pattern and the mouth opened during the embryonic and free embryonic phases.
Key Words
Breeding in captivity, reproductive behavior, ontogeny, Osteoglossomorpha
Introduction
The osteoglossiform family Notopteridae comprises four genera, eight better known and ten nominal species with mostly long and notably slender bodies occurring in tropical Africa and South and South-East Asia (
Reports on the reproduction of N. notopterus and notopterids in general are a bit heterogeneous and fragmentary in the literature. Courtship and spawning activity in N. notopterus was observed during the day (as also in our case) and at a temperature of 26-28 °C. This may last for about seven days according to
In the framework of a more extensive comparative study aimed at reproductive biology of osteoglossomorph fishes (
Materials and methods
A larger group of specimens of Notopterus notopterus originating from South-East Asia, had already been present at the Humboldt University of Berlin, before the onset of the study. These were nine females and five males with a total length of 198-269 mm and a total weight of 46 –159 g. The additional 20 specimens (10♀, 10♂) were purchased from a wholesale dealer (Aquarium Glaser in Rodgau, near Frankfurt am Main). This group consisted of individuals ranging from 178–248 mm in total length and 39–137 g total weight. Sexes of N. notopterus are distinguished by the shape of the genital papillae (
The fish were fed once a day on sliced beef heart and/or frozen chironomids.
Two tanks, one of 700 l and 400 l volume, respectively, were used to breed this species with sex ratios 2:1 and 1:1 (female/male). Each tank had been disinfected before usage. One breeding tank was equipped with black polythene shreds as plant imitation or hiding places and some flat large stones as spawning substrates, whereas the other tank was supplied with two large stones and sand, covering the entire bottom (Figs
The acclimatization period lasted about two to six months. This period of time was used for continuous observation of the selected specimens for breeding purposes, controlling aggressiveness and also to create the desired breeding conditions. During this phase, the fish established individual territories. Moreover, the male seemed to choose a female partner for courtship and mating.
Eggs, embryos and larvae were attained from ten spawning events. Directly after each spawning, the eggs were removed from the breeding tank and transferred into 20 × 10 × 6 cm plastic jars covered with a plastic lid or into Petri dishes measuring 5 to 10 cm in diameter. These were placed in a thermostat (27 °C, no aeration system) until the larvae started exogenous feeding. Larvae were fed for the first 7 days with fresh, newly hatched Artemia nauplii. Subsequently on the eighth day of feeding, food supply was substituted by older Artemia nauplii supplemented with small pieces of Tubifex. As the larvae grew, they were transferred into a small tank (20 × 20 × 20 cm) and provided with small pipes as hiding places. Once older than three months, the juvenile fish were additionally fed with small pieces of beef heart twice a week or alternatively also with a mixture of sliced meat, fish, shrimp, and paprika bound by gelatin.
Variation of environmental parameters to induce the gonad maturation was stimulated with the method developed by
The gonad’s maturity coefficient (MC) of dissected specimens measured as an index value (gonad weight/ total weight-total gonad weight) × 100, was used to verify and evaluate the effect of environmental factors on gonad development.
All photos of fertilized eggs, developmental stages of the embryo and larval development were made with two different cameras: a Leica S6E binocular fitted with a Canon PC1048 micro-camera or a Canon Powershot S50, digital camera mounted on a Leica L2 Stereomicroscope. Pictures taken from the laboratory showing the representative juvenile and adult fish as well as their tanks and in situ condition were photographed using a Canon EOS 350D digital Camera. Standard detailed photographs and measurements were always taken of anesthetized specimens, using MS222 (Tricain-Methane-Sulfonate), buffered with Sodium-Bicarbonate to maintain neutral pH. Extensive 24 hrs life-stream filming of behavior was done with a webcam (AXIS 2120 Network Camera) and with dim red-infrared-lighting during the night, sequences captured on a Dell Optiplex Workstation. The program for collecting sequences of 5 second intervals was developed by Dirk Striebing (Museum für Naturkunde) for remote observation and documentation projects. All handling of the fishes conformed the laws and rules of the TSchG in the latest versions of May 18, 2006 and July 28, 2014, and to come nearer to natural environmental conditions particular care was taken to avoid any disturbing actions, accidents, injuries or serious intraspecific aggressiveness.
The terminology of early ontogeny which was applied here is based on the concept of Balon, developed in a series of publications (
Results
1. Reproduction
A high number of successful breedings in captivity have been recorded that, if not prevented by forming larger groups, had left us almost with a marketable stock of Notopterus notopterus. The first two experiments are documented here in more detail. The first breeding experiment was performed with one male and two females. The method of variation of conductivity was based on experiments with polypteriforms, mormyrids and gymnotiforms (
First time spawning occurred on day 198 of the experimental period, followed by 19 spawning events with irregular intervals (see Table
The second breeding experiment comprised one male (TL = 261 mm, TW = 110 g; initial data at start of the experiment) and one female (TL = 236 mm, TW = 83 g) (Fig.
Courtship behavior was regularly observed throughout the entire daytime. Overall there were five steps of courtship behavior observed during these breeding experiments: 1) The male repeatedly followed or swam alongside the female, but still the female often avoided the male, 2) Male touched the female’s belly with his mouth several times while the female actively swam up and down in front of the male, 3) Male quivered the female by swinging rapidly his tail against the side of the female’s body, to which the female reacted by swimming quickly to the other side of the tank and then returning directly to her previous position, 4) A male and a female stayed still in a corner of the tank until the male suddenly swam to the other side, 5) The male later on was actively approached by the female and led the female to the spawning site.
In the particular case of the second breeding pair, mostly during this intense courtship behavior the male was actively seen preparing a suitable spawning site, by removing gravels and cleaning the spawning site with his mouth.
The 20 successful spawning events of the first breeding experiment were observed during day time. The pair seemed ready to spawn as the female showed a swollen belly and the male was apparently attracted by the female. Soon after the female had laid some eggs on the preferred substrate, the male quickly fertilized the eggs while the female was still nearby. When spawning was finished, the male remained at the spawning site, while the female left for the other side of the tank. The genital papilla of the female appeared bigger than usual during courtship and spawning. It is approximately 5–7 mm in length then.
Regarding most of the spawning events of the first breeding pair, nearly all the eggs were spawned on the same substrate and at the same location. The eggs were attached to the bottom side of larger stones. Nevertheless, in the last two spawning events of this breeding pair, eggs were found outside of the common area: on the edge of the filter (Fig.
In N. notopterus only the left gonad is developed. For the purpose of evaluating gonad maturation in relation to size, external shape of the belly, and potential external triggers of reproduction, the in situ conditions of dissected grown females and males were studied. Two females presenting an immature and a mature ovary, do show extremes of the maturity coefficients (MC) of 0.68 and 8.46, respectively. The single ovary in female N. notopterus is positioned laterally of the coiled intestine in the abdominal cavity on the left side of the fish. The immature ovary contains mostly oocytes in stadium 1 with their very dominant whitish color. The mature gonad of a female of 23 cm total length and 111.5 g weight is yellowish orange, occupies almost all of the space in the abdominal cavity, shows oocytes in different advanced stages and is surrounded by fat tissue.
As a whole the MC in 16 sacrificed and dissected specimens of N. notopterus was determined from grown specimens of the P and F1 generation, and did not show any correlation with the measured potential external factors of water quality, kept fairly constant in the aquaria.
Maturity coefficient (MC) values of the specimens used in breeding experiment 1 at the end of the experimental period were 8.46 (♀1), 8.75 (♀2) and 1.12 (♂1), at 26°C and decreasing conductivity (500 µS/cm) after a rise to 650 µS/cm corresponding to Fig.
Diagram of the course of the tested environmental factors: specific conductivity (C) and temperature (T) during 339 days in breeding group and tank I containing two females and one male of Notopterus notopterus. 20 spawnings of the female No. 1 were observed within a 5-month period. The breeding experiment was started after 3 months of acclimatization period. A swollen belly of the female was first observed after one month (arrow). * Asterisks refer to observed spawnings.
Course of the tested environmental factors during 331 days: conductivity (C), water level (WL) and temperature (T) in breeding tank II containing one female and one male Notopterus notopterus. Irregular spawning intervals were observed for 6 times within a 6-month experimental period. The breeding experiment was started after 40 days of acclimatization. Note the first swollen belly of the female (Arrow). *Asterisks refer to observed spawnings.
Overview of spawning events in breeding group 1 (2♀, 1♂) and breeding group 2 (1♀, 1♂) comprising number of eggs and control of pH value per individual spawning.
| Breeding group 1 | Breeding group 2 | ||||||
|---|---|---|---|---|---|---|---|
| Spawning Nr. | Spawning date | Eggs # | pH | Spawning Nr. | Spawning date | Eggs # | pH |
| 1 | 04.01.2010 | 50 | 8,5 | 1 | 16.12.2009 | 87 | 6,5 |
| 2 | 11.01.2010 | 68 | 8,2 | 2 | 23.12.2009 | 75 | 7 |
| 3 | 19.01.2010 | 90 | 7,9 | 3 | 14.01.2010 | 50 | 8,2 |
| 4 | 21.01.2010 | 50 | 7,7 | 4 | 17.02.2010 | 47 | 7,8 |
| 5 | 02.02.2010 | 50 | 7,7 | 5 | 28.02.2010 | 45 | 7,6 |
| 6 | 03.02.2010 | 25 | 7,7 | 6 | 15.06.2010 | 51 | 6 |
| 7 | 16.02.2010 | 74 | 7,5 | ∑ | 355 | ||
| 8 | 17.02.2010 | 30 | 7,5 | ||||
| 9 | 25.02.2010 | 105 | 7 | ||||
| 10 | 02.03.2010 | 225 | 7 | ||||
| 11 | 12.03.2010 | 113 | 6,5 | ||||
| 12 | 17.03.2010 | 27 | 6,5 | ||||
| 13 | 23.03.2010 | 120 | 6,7 | ||||
| 14 | 28.03.2010 | 40 | 7,3 | ||||
| 15 | 29.03.2010 | 180 | 7,3 | ||||
| 16 | 04.04.2010 | 70 | 7,6 | ||||
| 17 | 13.04.2010 | 15 | 7,9 | ||||
| 18 | 10.05.2010 | 118 | 8,3 | ||||
| 19 | 17.05.2010 | 30 | 8 | ||||
| 20 | 25.05.2010 | 80 | 7,7 | ||||
| ∑ | 1560 | ||||||
Spawning sequence of Notopterus notopterus during night time. Picture sequences selected from a one hour period of observation from automatized Webcam recordings with red to infrared-light. Egg deposition occurred in different positions, while the male accompanied the female in every action. (a) The male pushed gently the female’s belly with its mouth to the desirable spawning substrate; (b) both fish were seen floating very close to each other after the male fertilized the eggs; (c) the second spawn of the female followed soon after first fertilization by the male; (d) male pushes the female again to another substrate near the first location of spawning; (e) the male immediately fertilizes the eggs; (f) the female deposits eggs for the last time in this series.
Maturity coefficient (MC) of dissected Notopterus notopterus, as related to total length (TL), total weight (TW), and total gonad weight (TGW) data. Bold figures give values that correspond to those of breeding pairs.
| No | Sex | TL (cm) | TW (g) | TGW (g) | MC |
|---|---|---|---|---|---|
| 1 | ♂ | 23,7 | 129,94 | 0,45 | 0,34 |
| 2 | ♂ | 23,7 | 115,44 | 0,77 | 0,67 |
| 3 | ♂1 | 25,0 | 113,6 | 1,26 | 1,12 |
| 4 | ♂ | 27,2 | 170,4 | 1,26 | 0,74 |
| 5 | ♂2 | 26,3 | 144,17 | 1,09 | 0,76 |
| 6 | ♂ | 26,0 | 156,84 | 0,83 | 0,53 |
| 7 | ♂ | 25,5 | 161,64 | 1,18 | 0,73 |
| 8 | ♀ | 24,2 | 124,37 | 5,68 | 4,8 |
| 9 | ♀ | 25,2 | 131,47 | 7,44 | 5,9 |
| 10 | ♀ | 24,5 | 133,8 | 6,54 | 5,13 |
| 11 | ♀1 | 27,0 | 111,05 | 8,67 | 8,46 |
| 12 | ♀ | 21,6 | 82,15 | 2,76 | 3,47 |
| 13 | ♀ | 22,8 | 108,39 | 5,63 | 5,47 |
| 14 | ♀2 | 23,7 | 110,8 | 6,03 | 8,75 |
| 15 | ♀ | 22,1 | 112,4 | 7,64 | 7,29 |
| 16 | ♀3 | 23,3 | 118,64 | 8,3 | 7,52 |
2. Early Ontogeny
Fertilized eggs are adhesive, yellowish, and spherical with 3.8–4 mm in diameter. The egg envelope has many external ridges which are centered around the micropyle (Figs
Three spawned eggs of Notopterus notopterus. (a) The egg envelope has many spiralling ridges originating from the micropyle; (b) the micropyle (m), located above the animal pole, is clearly marked as an opening in the egg envelope of the ovulated egg; (c) view of an egg from the vegetal pole. Scale bar = 1 mm.
2.1 The embryonic period
The cleavage phase
Stage 1: zygote-one cell. At 1:10, a discrete and distinct brownish pattern emerged to the top of the egg, the blastodisc as characteristic of bipolar differentiation. Beneath the blastodisc, the cytoplasm strictly joined to the yolk due to the formation of the prospective yolk syncytial layer (Fig.
Stage 2: two-blastomere. At 2:00, the earliest cleavage furrow divides the blastodisc into two identical blastomeres, the two-cell stage (Fig.
Stage 3: four-blastomere. The next cleavage furrow yields four equivalent blastomeres and is situated in a right angle to the first cleavage furrow (Fig.
Stage 4: eight-blastomere. The third cleavage occurs at 2:42 and produces eight blastomeres, arranged in two parallel rows of four cells each (Fig.
Stage 5: early morula – 16 blastomeres. The furrows of the fourth cleavage are oriented roughly horizontally to oblique (Fig.
Stage 6: late morula. The fifth cleavage occurs at 4:30. The following cleavages develop into asynchronous formation of furrows producing unequal blastomeres in size and the germ comprises up to circa 32 blastomeres (Fig.
Stage 7: blastula. In specimens of this stage, at 6:25, the blastodisc shows a definite pebbled emergence and its upper exterior side forms a dome-shape. It is indeed distinguished from the yolk cell by a ring-like demonstrating the formation of the yolk syncytial layer (Fig.
Stage 8: flat blastula. The blastodisc divides into two types of cells at 7:40. The primary cell population recognized is the enveloping layer. It mostly originates from the superficial cells of the blastoderm that form an epithelial sheet with a mono-cellular layer. The deep layer of cells is located beneath the internal surface of the enveloping layer (Fig.
Stage 9: late blastula. At this stage the deep cells have multiplied producing a multicellular layer, observed at 8:35 to 9:00. As cleavage proceeds, cell size indeed declines while the cell number enhances leading to an advanced blastula in the limited blastodisc area. The blastoderm becomes opaque and firm. It also shows a broad and thick belt of the external yolk syncytial layer. The animal surface of the yolk cell underlying the blastoderm is flat and most of the yolk vacuoles are located inside. Shortly afterwards, the periphery of the blastoderm extends over the adjacent yolk margin (Fig.
Cleavage in Notopterus notopterus and blastula formation. (a) Stage 1: one cell (zygote) with forming blastodisc (bd), 1h:10min; (b) Stage 2: two blastomeres (b), at 2:00h; (c) Stage 3: four cells, 2h:20min; (d) Stage 4: eight cells, 2h:42min; (e) Stage 5: early morula with 16 cells, 3h:45min; (f) Stage 6: asynchronous blastomeres up to circa 32 cells, 4h:30min; (g) Stage 7: blastula with compact knob-like blastodisc, 6h:25min; (h) Stage 8: flat blastula with expanding yolk-sac syncytium (ys), roundish mound of the blastoderm on the top of the yolk, 7:40; (i) Stage 9: late blastula, the surface of blastoderm appears smooth, covering part of the yolk, but the cells are still distinct, 8h:35min. Scale bar = 1 mm.
The embryonic phase
Stage 10: epiboly, evacuation zone. Animal pole and vegetal pole are clearly separated from each other, this occurs around 16:00 h. The germ acquires again the circular shape with a lens-shaped blastoderm. The upper part of the yolk is densely packed with globules. The blastoderm covers 30 % of the yolk surface and the margin of the blastoderm is lying just beyond the lower margin of the external yolk synctial layer. The inflated evacuation zone is covered by a single thin and clear mono-layered epidermal enveloping layer. The animal surface of the yolk sphere is flat. The internal yolk syncytial layer gets more prominent beneath the extremely thin and translucent blastoderm (Fig.
Stage 11: 50% epiboly. The blastoderm spreading and covering 50 % of the yolk surface. As the blastoderm extends vegetally, the external yolk syncytial nuclei move underneath the blastoderm to distribute over the yolk mass. The margin of the blastoderm can be distinguished from the germ ring (Fig.
Stage 12: embryonic shield. The margin of the blastoderm further spreads over the yolk margin and a visible embryonic shield emerges as a slender bulge. The longitudinal axis of the embryonic shield will be later on recognized as the prospective embryo. The evacuation zone seems to be dislocated from the vertical axis of the yolk mass (Fig.
Stage 13: early neurula. The embryonic shield becomes elongated towards the animal pole. It is formed by a thickening of the ectodermal epithelium to form a classical neural plate. A small evacuation zone is still present, but is not seen again after this stage (Fig.
Stage 14: 75% epiboly and notochord-formation. The blastoderm has covered about 75 % of the yolk surface by the end of this stage. The neural folds of the prospective head region are elevated from the epidermal yolk sac cover. A notochord can be seen already at the midline of the neural plate and this morphologically defines the rostral-caudal axis of the future embryo. The first three somites are also visible from this stage onward (Fig.
Stage 15: wedge-shaped neural plate. The blastoderm expands spreading and covering up to almost 90 % of the yolk surface, with the yolk plug obviously much reduced from the previous stage. The neural plate continues to extend laterally and becomes wedge-shaped (Fig.
Stage 16: latest epiboly. The blastoderm covers almost the entire yolk surface, leaving an exposed small yolk plug. The germ ring is now swollen all around. The primary yolk sac cavity extends further rostrally and caudally underneath the entire neural plate to form the segmentation cavity (Fig.
Stage 17: spoon-shaped embryo. Epiboly is completed and the yolk mass is wholly wrapped with both the blastoderm and the yolk syncytial layer. As neurulation proceeds, a neural groove becomes evident along the midline of the plate, with a widened, spoon-shaped depression at the anterior end. Shortly afterwards the neural folds have approached each other along the midline, which causes the neural plate to take a dumbbell-shaped appearance. In the prospective head region, the neural folds thicken (Fig.
Stage 18: early trunk-tail bud. The lateral edges of the neural plate are prominent and fold in toward the midline of the embryo, mainly in the prospective trunk-tail region. The majority of the caudal portion of the neural anlage and underlying mesoderm project from the epidermal yolk sac cover to form a rudimentary trunk-tail region. The embryo now has around 10–13 somites. The earliest somatic muscular activity occurs in the posterior trunk-tail region. The observed contractions were mostly weak lateral trunk-tail flexions. A first twitch resulting from touching the embryo was observed, which was mostly followed by a secondary reaction of several trashes of the tail (Fig.
Stage 19: tail bud-bent. This stage is characterized by a 90° bend of the free trunk-tail. The somites and the mesoderm destined for the further differentiation of somites are in contact at the midline above the spinal cord, and the mesodermal matter of the lateral plates in the head region grows dorsally. The embryo now has 19–20 somites. The observed contractions were still mostly weak lateral trunk-tail flexions. Any disturbing action nearby will apparently provoke a twitch, which is then followed later on by a secondary backlash or even several thrashes of the tail. The heart tube is visible. The caudal tail region of the embryo, in which Kupffer’s vesicle is located, is separated from the yolk sac and curved downward along the curvature of the egg envelope (Fig.
Stage 20: otic placode and heart-beat. The otic placode emerges for the first time at this stage. Contractions of the heart are observed. The straightening of the embryo and the presence of blood cells enables now to analyze the blood circulation in more detail. The yolk sac is covered by a dense plexus of anastomosing subintestinal venae vitellinae. Spontaneous, vigorous, flexing movements of the body are common, which cause the embryo to rotate within the egg envelope. If illumination is changed, contraction frequency is increased for a short time. The central nervous system lays in a deep groove formed by the remarkably elevated paraxial mesoderm in the trunk-tail region and the prominent, densely packed mesoderm mass in the head region. The forming heart is seen inside the extended pericardial cavity. The blood cells are circulating through a system comprising in a sequential order: a two chambered heart, a dorsal aorta from the heart to the posterior limit of the yolk, a short intestinal loop, a subintestinal vitelline vein to the yolk sac periphery, and after taking a diffuse random course across the external surface of the yolk sac back to the heart. (Fig.
Stage 21: fin-fold. The first sign of the embryonic fin fold is observed here. It runs along the midline from the first somite caudally and encircles to the entire trunk-tail end. The trunk and tail region constantly perform thrashing movements. There are about 28 somites visible at this stage. The dorsal aorta is well discernible in the anterior body region. The entire head region has significantly enlarged and shows several new features. A lens is formed inside the eye cup, when the choroid fissure is still wide open. No pigment is discerned in the eye yet. The lobi lineae lateralis become visible in the brain (lll).The yolk sac is covered by a dense plexus of anastomosing subintestinal venae vitellinae. The membranous embryonic fin fold becomes conspicuous in intense illumination (Fig.
Stage 22: eye pigment. Diffuse pigmentation appears in the eye. A few small unbranched melanophores that contain diffuse melanine pigment are distributed all over the ectoderm of the head already. The muscular contractions of the embryo have changed into powerful movements of the entire body by now. The vascular fin fold network extends over the entire fin fold. Some larger venae vitellinae are established at the lateral region of the yolk mass surface. 48-52 pairs of somites were counted (Fig.
Stage 23: pre-hatching. The yolk cavity is enlarged beyond the entire anterior part of the embryo still attached to the epidermal yolk sac cover. The anterior part of the head is slightly undercut at its juncture with the epidermal yolk sac cover. At quiescent conditions the tip of the curved trunk-tail region reaches the vertical end of the head. This tip may touch or even pass over the head during movements. The embryo performs strong vigorous rotations and quivering motions. These movements are sufficient to change the orientation of the embryo inside of the egg envelope. The two columns of the presumptive corpus cerebelli fuse dorsally in the midline, forming also a transversally widened bulge behind the fissure rhombo-mesencephalica. This bulge curves forwards, inwards, ventrally and also grows caudally, forming the primitive cerebellar cavity. The lobi lineae lateralis are very obvious and start to elevate. The embryo is in a twisted position inside the egg-envelope due to its elongated body (Fig.
Continuation of epiboly and neurulation in Notopterus notopterus. (a) Stage 10: evacuation zone (ez) at animal pole and the deepening of the marginal zone (mz) between the animal pole and the vegetal pole, 19h:11min; (b) Stage 11: 50 % epiboly, germ ring (gr) positioned in between of animal pole and vegetal pole, 25h:15min; (c) Stage 12: embryonic shield (es) on the animal pole, note the micropyle (m) and the translucent evacuation zone (ez), 27h:00min; (d) Stage 13: onset of neurulation with vertical view, neural plate (npl), 29h:15min; (e) Stage 14: 75 % epiboly on vertical view, note the yolk plug is mostly constricted by the flat and short germ ring (gr), 36h:00min; (f) Stage 14: transversal view, note the rostral (r) and caudal (c) part of the embryonic shield can be distinguished; (g) Stage 15: 90 % epiboly; note the reduced yolk plug (yp), 39h:10min; (h) Stage 16: latest epiboly, note the tiny remnant of the yolk plug (yp), vertical view; (i) Stage 16: transversal view showing the wedge-shaped neural plate and the rostral (r) and caudal (c) part of the embryonic shield. Arrowhead points to notochord 41h:35min. Scale bar = 1 mm.
Embryonic development in Notopterus notopterus. (a) Stage 17, spoon shape, 48:50; nf- neural fold in the tail region; (b) Stage 18: early trunk-tail bud, note the remnant of the evacuation zone, 51h:05min; note somites (som) and the tail bud (tb) at the posterior part; (c) Stage 19: tail-bud bent; Kupffer’s vesicle (Kv) appears in the bent tail bud, 84h:00min;cc - corpus cerebelli around the rhombencephalic area; (d) Stage 20: otic placode (op) and heart beats (h) observed, 92h:25min; (e) Stage 21: fin-fold stage, 95h:25min. Note the lobilineaeliteralis (lll), the otic placode (op) and the embryonic fin fold (ff). (f) Stage 22: eye pigment (e), 122h:05min; note the broadened fin fold (ff) along the body; (g) Stage 23: “C” shape, pre-hatching embryo; note the edge of the caudal fin, and the lobilinealateralis (lll). 146h:50min. Scale bar = 1 mm.
The eleutheroembryonic phase
Stage 24: hatchling. Hatching starts at about 168-204 hrs, 7 to 8 and 1/2 day after spawning and the free embryos measure10.5 mm. Despite constant temperature, hatching is variable among different breeding events and even among the same egg cluster and may be a process of several minutes to half an hour duration. The egg envelope has lost its stability, which could be seen by its pliable texture. The tail usually protrudes first and after a partial rupture of the egg envelope, the free embryos with the head part often still remain within the egg envelope for some time. The yolk sac measures around 3.8 mm in total length at this stage, often situated still inside the egg envelope. As the embryonic fin-fold is upright now, the development of the dorsal fin can be followed from this stage onward (Fig.
Stage 25: completed rupture. The egg envelope is completely ruptured and the embryo is free. It usually takes around 20 minutes until the embryo successfully detached from its egg envelope. Shortly after hatching, the embryos rest calmly and straight on the ground. The eyes are completely black. The tiny, hemispherical pectoral fins project from the dorsal epidermal yolk sac cover, and are positioned close to the heart region. The yolk sac still measures around 3 mm. There are 58–62 pairs of somites observed at this stage (Fig.
Stage 26: jaws and branchial arches. The head still remains bent downwards along the yolk sphere. The first mesenchymal condensations of jaws and branchial arches can be seen between head and yolk mass. Usually the yolk mass is no longer rounded, but has become egg shaped with a narrow posterior end. The yolk sac measures around 2 mm and the free embryos usually measure 11 mm at this stage. When the freshly hatched embryo is stimulated, it makes rapid, striking movements through which it whirls around in circles with the yolk-sac as a center of gravity. The early free embryos are still too heavy for directional movements (Fig.
Hatching process and the free embryonic stages in Notopterus notopterus. (a) Stage 24: just hatched-embryo after breaking the weak egg envelope, caudal part is relieved, anterior part still stuck in the remnant of the egg-envelope, note the emergence of dorsal and caudal fin anlage; yolk sac (yc) still covered with egg envelope, 168h:05min; (b) Stage 25: rupture the complete egg-envelope and free anterior part of the embryo, 168h:25min; note the tiny pectoral fin bud (black arrowhead); (c) Stage 26: jaw and branchial arches formed, day 8; (d) Stage 27: mouth opening, day 10; note the emergence of the swim bladder vesicle (sb); white arrowheads point to gradual development of dorsal fin. Scale bar = 5 mm.
The Protopterygiolarval phase
Stage 27: mouth opening. Some evident changes in the outer appearance and in the structure of the head can be easily seen. The head process has already undergone a significant lift. Several structures are more clearly defined. In particular, the heart is beginning to descend inside the pericardial cavity. The emergence of gills starts close from here. The anlagen of the dorsal and caudal fins appear simultaneously as denser concentrations of mesenchyme in the fin fold. The formation of the first rays in the caudal fin is evident from this stage onward. The future swim bladder can be identified. Melanophores spread out significantly not only on the head part but also appear for the first time on the trunk and tail part. The head lift and further growth and straightening of the head exposes the underside of the head. The lower jaw has straightened forward. Mouth opening shows a “>” shape with clear separation of upper and lower jaws. The central lepidotrichial rays of the caudal fin start to segment. The yolk sac measures around 1.4 mm and the embryos measure 12 mm now (Fig.
The tiny pectoral fin bud becomes ellipsoid in shape due to its dorso-caudal prolongation, a detail seen in Fig.
Stage 28: progressive median fin-fold regression and formation of distinct fins. The dorsal and caudal fins lengthen over the embryonic fin fold. The continued regression of the dorsal median fin fold allows determining the contour of the body, the dorsal, anal, and caudal fins. The pectoral fin anlage now is circular with an enlarged and indented rim. The haemal and hypural processes are covered by a few muscles. From this stage onward, the embryos become increasingly more mobile and are apparently attracted by accumulations of food organisms. The embryo is now capable of completely lifting its head and actively closing or opening its mouth. The upwards flexion of the end of the notochord has reached its final shape, perpendicular to the longitudinal axis of the embryo. The embryo measures 13.6 mm and the capacity of the yolk-sac has decreased significantly (Fig.
Stage 29: late embryo. The dorsal embryonic fin fold has significantly shrunk in height, especially in the rostral part. The late embryos measure 14.9 mm. The yolk sac remains visible. The pectoral fins are now formed with a proximal bud and a fan supported by 6 segmented lepidotrichial rays each. They are functional and used as in many other teleostean larvae for directional propulsion. In this stage, first movements of active breathing of the gill cover are observed (Fig.
Stage 27, day 9; larger magnification (see also Fig.
Late embryonic and larval development in Notopterus notopterus. (a) Stage 28: pronounced regression of median fin fold, day 12; note almost completely reduced yolk sac (ys);pectoral fin, arrow; (b) Stage 29: late embryonic phase day 14, pectoral fin buds of both sides seen; (c) Stage 30: beginning of larval period, extrinsic feeding, developed and gas-filled swim bladder, day 17; (d) Stage 31: pigmented iris and cornea are very distinct, day 24; (e) Stage 32: anal and caudal lobe formation, day 36; arrowheads point to the leading edge of the gradually developing dorsal fin. Scale bar = 5 mm.
2.2 The larval period
The pterygiolarval phase
Stage 30: exogenous feeding. The first stage of the larval period is characterized by exogenous feeding concurrent with endogenous nutrient-utilization. The yolk sac remnant is still present but almost resorbed. The swim bladder appears tube-shaped and gas-filled and continues to lengthen from this phase onward. The dorsal fin has grown showing newly developed fin-rays. The final shape of the caudal fin is reached by the conspicuous constriction and regression of the remaining dorsal fin fold at the caudal end of the peduncle. Melanophores spread over the integument. Pectoral fins are well developed and very mobile. The depicted individual larva at this stage measures 16.2 mm (Fig.
Stage 31: eye differentiation. The swim bladder starts to elongate dorso-rostrally to connect with the otic vesicle. The yolk sac remains are now completely resorbed. From this stage onward, the larvae are very mobile and actively hunting for food. The pigmented cornea of the eye has developed, the iris contrasts jet-black, eye movements are seen and the larvae are swimming closely along the bottom. The swim bladder has elongated a bit more and appears thinner posteriorly. An intense yellow-orange mass fills the gut, representing captured Artemia nauplii. The total length of the depicted individual larva is 21 mm (Fig.
Stage 32: anal and caudal lobe formation. The dorsal fin separates from the peduncular fin fold remain and the asymmetry of the fan of the caudal fin has become more conspicuous. The high number of lepidotrichia becomes distinct in the anal fin-fold, confluent with the caudal fin. The larvae usually measure 24 mm or more at this stage (Fig.
Development of dorsal, caudal and pectoral fin are almost synchronous and precede the differentiation of lepidotrichia in the anal fin-fold. There is virtually no pre-anal fin fold and the elongate embryonic analis simply grows and is supplemented with ossifying lepidotrichia, radials and a differentiated musculature. From the last larval stage 32 onward, the characteristic undulatory movements of the anal fin are performed. Still later the tiny pelvic fins appear.
2.3 The juvenile period
The juvenile period of Notopterus notopterus comprises five stages as follows:
Stage 33: juvenile – 1. This stage can be clearly characterized as a transition mark from the larval period to the juvenile period by its profound change in the pigmentation pattern. The embryonic fin folds are no longer visible. The swim bladder has extended caudally and is densely covered by melanophores. The lateral line of the body becomes visible, starting right behind the head in the opercular region at this stage. The formation of tube-like anterior nostrils is also starting from this stage onward. The body grows significantly in height and melanophores become denser also in the caudal part. Silvery-colored scales appeared on the midline of the anterior part of the trunk and at the base of the pectoral fin and caudal fin. The expansion of the stomach and the intestine can be clearly seen. The typical early juvenile measures now 29 mm (Fig.
Stage 34: juvenile – 2. At this stage the body has fully changed its color into “flamed” and roughly vertically or ventro-cranially to dorso-caudally arranged dark brown stripes. There is a bit of black color at the outer, the corneal part of the eye. This typical juvenile-2 measures 34 mm (Fig.
Stage 35: juvenile – 3. The body shows much stronger dark brownish stripes and pronounced elongation of tubular nostrils is apparent at the snout. The genital papilla emerges for the first time. The depicted individual measures 46 mm TL (Fig.
Stage 36: juvenile – 4. There is once again an obvious change in the pigmentation pattern as compared to the previous stage. The striped, dark-brown coloration has disappeared. The whole body turns into bronze-gold and the scales are externally well visible from this stage onward. The pale remains of the dark brown stripes can still be recognized. The pelvic fins start to emerge at this stage and the depicted individual measures 76 mm TL (Fig.
Stage 37: juvenile – 5. The dorsal fin loses its trapezoidal shape and forms into the triangular shape of the adult. Uniformly golden-brown now, the specimens have attained the general body profile of the immature adult now. The genital papilla is clearly recognized behind the elongated pelvic fins. The depicted late juvenile individual measures 145 mm TL (Fig.
Sequence of development of the dorsal fin starting from hatching until the end of the larval period in Notopterus notopterus. (a) shortly after hatching, day 7, first mesenchyme condensation; (b) dorsal fin bud appears, day 8; (c) distinct dorsal fin bud and regression of embryonic fin fold, day 12; (d) well-developed dorsal fin anlage with 9 distinct radials and muscles, 7 fin rays, 17 days; (e) differentiated dorsal fin and regressed fin-fold of a stage 31 larva, 24 days; (f) dorsal fin in transition to a juvenile, 8–9 differentiated lepidotrichia, 36 days. Also note the progressive folding of myomeres and strengthening of myosepta; arrowhead points to developing dorsal fin. Scale bar = 2 mm.
Sequence of caudal fin development starting from hatching until the larval period in Notopterus notopterus. (a) shortly after hatching, day 7; (b) hemal spines and hypural plates (hh) emerged; note the slight regression of fin fold, day 10; (c) fin fold dorsally relatively lower, but intact, caudally completely replaced by 10 caudal fin rays, pterygiolarval phase, stage 30, day 20; (d) well-developed caudal fin in an early juvenile stage 33 in perfect continuity with the anal fin, day 36;Cfm – caudal fin mesenchyme; ff- fin folds; cfr – caudal fin rays; cf – caudal fin; af – anal fin. Arrows indicate the approximate border between caudal and anal fin. Scale bar = 2 mm.
Juvenile transformation in Notopterus notopterus. (a) Stage 33: early juvenile in larger magnification, note the appearance of lateral line and short tubular nostrils, day 52; (b) Stage 34: stripe colored body of juvenile, day 70; (c) Stage 35: accentuated stripe pattern alongside the body, day 80; arrow points to the base of the developed translucent pectoral fin, barely visible; white arrowheads show the anterior nostrils; black arrowhead points to the genital papilla. Scale bar = 10 mm.
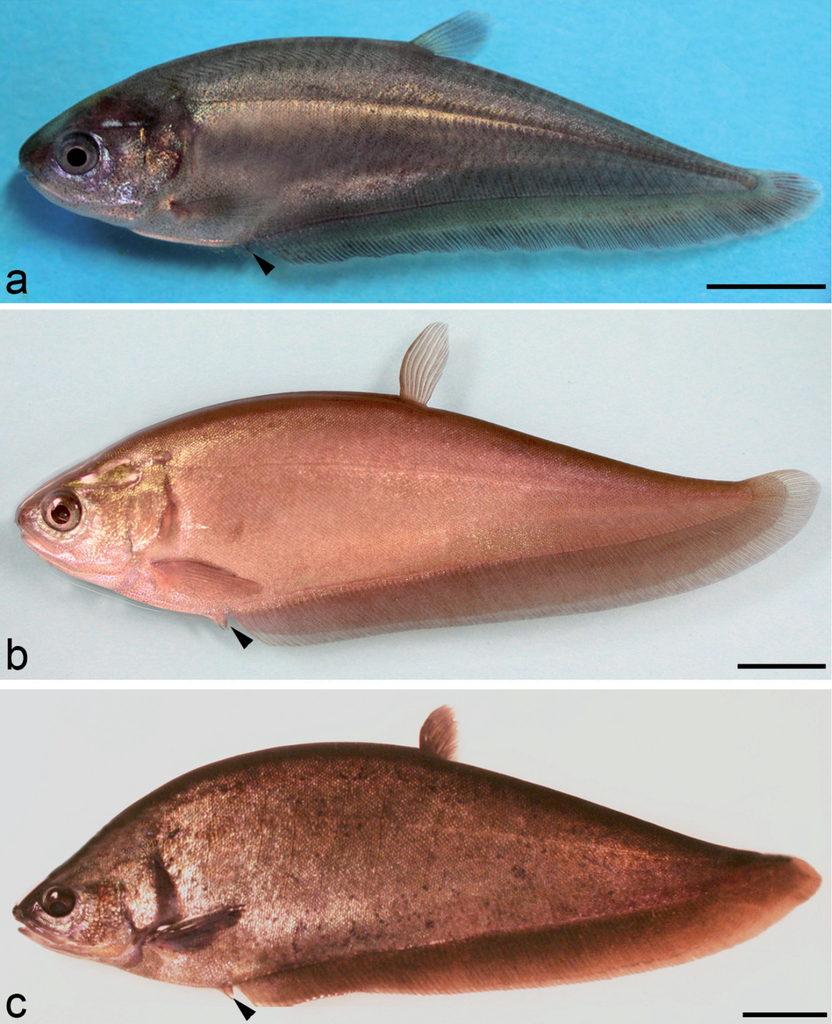
Late juvenile to the maturation stage in Notopterus notopterus. (a) Stage 36: appearance of ventral fin, black arrowhead, day 92; (b) Stage 37: late stage of juvenile with total length of 145 mm, note the change in color and the emergence of scales, 18 months; (c) Stage 38: adult male with total length 275 mm, captured at age of 30 months; note the ventral fin (arrowhead) longer than the genital papilla. Scale bar = 10 mm (a); 20 mm (b); 30 mm (c).
Development of the genital papilla of a female Notopterus notopterus, seen in various photographs at high magnification. (a) The emergence of genital papilla, juvenile, stage 35, total length (TL) 52 mm, day 83; note the pair of ventral fins; (b) genital papilla of a stage 36 juvenile with TL 100 mm, 5 months; (c) stage 36, TL=124 mm, 8 months; (d) stage 36 TL= 136 mm, 12 months; (e) stage 37, TL=145 mm, 18 months old specimen; (f) Mature genital papilla obviously longer than the pelvic fins, TL= 230 mm, 24 months old adult female; significant growth of the genital papilla (white arrowhead) in between the ventral fins (arrow) and anal fin. Scale bar= 2 mm (a, c, e); 5 mm (b, d, f)
2.4. The adult period
Stage 38: adult. The head shape and body pigmentation are different from the late juvenile. From this stage onward, the genital papilla of the mature fish can be easily differentiated between male and female by its form. The female’s genital papilla is whitish, broader and shorter, whereas the male’s is yellowish, thinner and longer. As turned out with experience, gonad maturation can be easily observed by the swollen abdomen and increase of appetite starting at the age of around 24 months and showing courtship behavior in 30 month old fish. A survey of all development stages in N. notopterus is presented in Table
Overview of developmental stages of Notopterus notopterus (27 °C), concept of determination of stages, phases and periods according to
| Period: Embryonic | Phase: | Stage | Age (hrs:min) | Characteristics | Figure |
|---|---|---|---|---|---|
| Cleavage | 1 | 1:10 | Zygote-1 cell | 9a | |
| 2 | 2:00 | 2 Blastomeres | 9b | ||
| 3 | 2:20 | 4 Blastomeres | 9c | ||
| 4 | 2:42 | 8 Blastomeres | 9d | ||
| 5 | 3:45 | Early morula | 9e | ||
| 6 | 4:30 | Late morula | 9f | ||
| 7 | 6:25 | Blastula | 9g | ||
| 8 | 7:40 | Flat blastula | 9h | ||
| 9 | 8:35 | Late blastula | 9i | ||
| Embryonic | 10 | 19:11 | Evacuation zone | 10a | |
| 11 | 25:15 | Epiboly 50% | 10b | ||
| 12 | 27:00 | Embryonic shield | 10c | ||
| 13 | 29:15 | Early neurula | 10d | ||
| 14 | 36:00 | Epiboly 75% | 10e-f | ||
| 15 | 39:10 | Wedge-shaped neural plate | 10g | ||
| 16 | 41:35 | Latest epiboly | 10h-i | ||
| 17 | 48:50 | Spoon-shaped | 11a | ||
| 18 | 51:05 | Early trunk-tail bud | 11b | ||
| 19 | 84:00 | Tail bud bent | 11c | ||
| 20 | 92:25 | Otic placode and heart beat | 11d | ||
| 21 | 95:25 | Fin fold | 11e | ||
| 22 | 122:05 | Eye pigment | 11f | ||
| Eleutheroembryonic | 23 | 146:50 | Pre-hatching stage | 11g | |
| 24 | 168:05 | Hatching stage | 12a | ||
| 25 | 168:25 | Completed rupture of egg envelope | 12b | ||
| Protopterygio“larval“ | 26 | day8 | Jaw and branchial arches | 12c | |
| 27 | day10 | Mouth opening | 12d | ||
| 28 | day12 | Finfold regression | 14a | ||
| 29 | day14 | Late embryonic | 14b | ||
| Larval | Pterygiolarval | 30 | day17 | Exogenous feeding | 14c |
| 31 | day24 | Developed eye | 14d | ||
| 32 | day36 | Caudal and anal lobe | 14e | ||
| Juvenile | 33 | day52 | Juvenile 1 | 17a | |
| 34 | day70 | Juvenile 2 | 17b | ||
| 35 | day80 | Juvenile 3 | 17c | ||
| 36 | day92 | Juvenile 4 | 18a | ||
| 37 | 18months | Juvenile 5 | 18b | ||
| Adult | 38 | 30months | Adult | 18c |
Discussion and conclusions
Environmental factors in reproduction. Generally it shall be stated here that Notopterus notopterus is by no means a “sluggish” fish as sometimes stated with reference to the family Notopteridae. In contrast, we always found them alert and mobile, with a voracious appetite and latent aggressiveness.
There is no obvious reaction to the environmental factors (decreasing conductivity, constant water level and slight variation of temperature) in N. notopterus. A female of the breeding experiment 1 (two females and male), indeed showed a swollen belly before the onset of decreasing conductivity, according to our results a reliable external sign of gonad maturation. Intense courtship behavior observed until the first spawning occurred after six months experimental period and one month after drastically decreasing and again elevated conductivity to high levels of around 800 µS/cm at constant water level. In the following series of spawning activities, no correlation is observed between spawning acts and changing water temperature (23-27°C) or conductivity values (150-660 µS/cm). In the breeding group 2 comprising a pair of N. notopterus, swollen bellies as indication for gonad maturation and courtship behavior were seen before onset of aimed manipulation of the water quality and at rather high levels of conductivity between 450 and 650 µS/cm. Courtship behavior and spawning occurs in a wide pH-range too, between 6.0 and 8.5 (Table
Gonads and fractional spawning. N. notopterus possess a single gonad located on the left side of the body, as observed also in Xenomystus nigri and Osteoglossum (
The presence of both ovaries is also found in the Mooneye Hiodon tergisus (
Similar to what
Spawning, eggs and parental care. This study confirms that Notopterus notopterus is a substrate spawner with rather large eggs, a semitransparent adhering egg envelope and a yellowish yolk mass, devoid of oil globules. This is in accordance with
Preferred are stable, sheltering objects as a spawning site, like stones or even filter material and sand or gravel is actively removed by the male. Some simple sort of parental care is perfomed by the male too in aggressively protecting the eggs against intruders. Parental care was reported by other authors in Notopteridae (
Spawning in N. notopterus in our case occurs mostly during day time, with few observed exceptions.
In this study the number of eggs per spawning in N. notopterus varied from 15–225 eggs. Some previous authors reported number of eggs per spawning in Notopteridae between, 50 eggs (
Ontogenetic development. There is nothing very special observed and to be discussed in the earliest embryonic development up to hatching as compared to other teleostean fish and typically telolecithal eggs. There are, however, some interesting differences to literature data and discrepancies to an ideally saltatory development scheme that have to be noted. In this study it has been shown that N. notopterus hatched at 7 days (168 hrs) after spawning at a constant temperature of 27 °C, resulting in 4536 deg × hrs with some variability of several hours within the same egg-clutch.This was prolonged up to 8 and1/2 day (204 hrs) in lower temperature basins at 23°C (4692 deg × hrs). This is a bit different and mostly longer than what other authors found: 5–6 days after spawning at 26–28 °C (
In this study, free embryos of N. notopterus, directly after hatching, measured 10.5 mm in total length, which however, is much longer to what
A variable hatching size of embryos is often also reported in other osteoglossomorphs and teleost fishes, and the size at hatching is certainly influenced by environmental factors such as temperature and oxygen level (
According to our results and characteristics, hatchlings of N. notopterus are rather large and several morphological differentiations occur during the embryonic and eleutheroembryonic phase already as compared to several other known osteoglossomorphs and osteoglossiforms. Eye pigmentation in N. notopterus commences within the egg envelope when five or six days old. In contrast, in Pollimyrus isidori (
The development of pectoral, dorsal, anal and caudal fins starts prior to the onset of exogenous feeding in N. notopterus. This is similar to Pollimyrus isidori, P. adspersus (
Possessing a large yolk sac allows the embryos to instantly structure the whole suite of permanent organs without being required to modify any temporal larval structures (
Acknowledgements
We are grateful to Hartmut Hoefft, Wolfgang Bernau, Suzanne Grübel, Petra Grimm, Annett Billepp and Jutta Zeller for their help in the laboratory and animal keeping facilities of both institutions. Dirk Striebing arranged for the observatory and automated picture capture of courtship behavior. Dr. Salif Diedhiou and Linh Nguyen shared much of their practical experiences in fish breeding with us and provided useful ideas and discussions. Professor Mohammad Ali Azadi kindly provided a thorough review and erased some mistakes.
References
- Alexander RMcN (1966) Pysical aspects of swimbladder function. Biological Reviews 41: 141–176. https://doi.org/10.1111/j.1469-185X.1966.tb01542.x
- Amanze D, Iyengar A (1990) The micropyle: a sperm guidance system in teleost fertilization. Development 109: 495–500.
- Argumedo EGT (2005) Arawanas, manual para la cria comercial en cautiverio; Asociacion del Acuicultores del Caqueta (ACUICA). Florencia, Caqueta, 105 pp.
- Argumedo EGT (2009) Arawana Azul, manual para manejo de reproductores en cautiverio. Asociacion del Acuicultores del Caqueta (ACUICA). Florencia, Caqueta, 95 pp.
- Armbrust W (1964) Der Schmetterlingsfisch, Pantodon buchholzi, seine Pflege und Zucht. Aquarien und Terrarien Zeitschrift 17: 2–5.
- Axelrod HR, Burgess WE (1981) Spawning Notopterus notopterus. Tropical Fish Hobbyist 29(12): 36–42, 44–49.
- Azadi MA, Islam MA, Nasiruddin M, Quader MF (1994) Food and feeding habits of a featherback, Notopterus notopterus (Pallas) (Notopteridae : Clupeiformes) from the Kaptai reservoir. Chittagong University Studies Part II Sc 18(2): 183–190.
- Azadi MA, Islam MA, Nasiruddin M, Quader MF (1995) Reproductive biology of Notopterus notopterus (Pallas) in Kaptai reservoir, Bangladesh. Bangladesh Journal of Zoology 23(2): 215–220.
- Balon EK (1975a) Reproductive guilds of fishes: a proposal and definition. Journal of the Fisheries Research Board of Canada 32: 821–864. https://doi.org/10.1139/f75-110
- Balon EK (1975b) Terminology of intervals in fish development. J. Fish. Res. Board Can.32: 1663–1670. https://doi.org/10.1139/f75-196
- Balon EK (1979) The theory of saltation and its application in the ontogeny of fishes: Steps and thresholds. Environmental Biology of Fishes 4: 97–101. https://doi.org/10.1007/BF00005446
- Balon EK (1981) Additions and amendments to the classification of reproductive styles in fishes. Environmental Biology of Fishes 6: 377–390. https://doi.org/10.1007/BF00005769
- Balon EK (1984) Patterns in the evolution of reproductive styles in fishes. In: Potts GW, Wootton RJ (Eds) Fish Reproduction: Strategies and Tactics, Academic Press, London, 35–53.
- Balon EK (1999) Alternative ways to become a juvenile or a definitive Phenotype (and on some persisting linguistic offenses). Environmental Biology of Fishes 56: 17–38. https://doi.org/10.1023/A:1007502209082
- Bartsch P, Britz R (1997) A single micropyle in the eggs of the most primitive living actinopterygian fish Polypterus. Journal of Zoology, London 241: 589–592. https://doi.org/10.1111/j.1469-7998.1997.tb04850.x
- Battle HI, Sprules WM (1960) A description of the semi-buoyant eggs and early developmental stages of the Goldeye, Hiodon alosoides (Rafinesque). Journal of the Fisheries Research Board of Canada 17: 245–266. https://doi.org/10.1139/f60-020
- Blake BF (1977) Aspects of the reproduction biology of Hippopotamyrus pictus from Lake Kainji, with notes on four other mormyrid species. Journal of Fish Biology 11: 437–446. https://doi.org/10.1111/j.1095-8649.1977.tb04138.x
- Breder CM, Rosen DE (1966) Modes of reproduction in fishes. Natural History Press, New York, 941 pp.
- Bridge TW (1900) The air-bladder and its connection with the auditory organ in Notopterus borneensis. The Journal of the Linnean Society of London (Zoology) 27: 503–540. https://doi.org/10.1111/j.1096-3642.1900.tb00420.x
- Britz R (2004) Egg structure and larval development of Pantodon buchholzi (Teleostei: Osteoglossomorpha), with a review of data on reproduction and early life history in other Osteoglossomorphs. Ichthyological Explorations of Freshwaters 15(3): 209–224.
- Britz R, Kokoscha M, Riehl R (1995) The anabantoid genera Ctenops, Luciocephalus, Parasphaerichthys, and Sphaerichthys (Teleostei: Perciformes) as a monophyletic group: Evidence from egg surface structure and reproductive behavior. Japanese Journal of Ichthyology 42: 71–79.
- Day F (1889) Fish fauna of British India. Vol. I. Taylor and Francis, London, 548 pp.
- De Vlaming VL (1974) Environmental and endocrine control of teleost reproduction. In Schreck, CB (ed), Control of Sex in Fishes, Virginia Polytechnical Institute and University, Blacksburgh, Virginia, VPI-SG-74–01, 18–23.
- Diedhiou S, Moritz T, Bartsch P, Kirschbaum F (2007a) Comparison of Pollimyrus isidori and Pollimyrus adspersus (Mormyridae) based on morphometric, meristic, ontogenetic, and physiological characteristics. Bulletin of Fish Biology 9: 61–88.
- Diedhiou S, Bartsch P, Kirschbaum F (2007b) The embryonic and larval development of Pollimyrusisidori (Mormyridae, Osteoglossomorpha): It’s staging with reference to structure and behaviour. Bulletin of Fish Biology 9: 61–88.
- Eschmeyer WN, Fong JD (2017) Species by family and subfamily. http://researcharchive.calacademy.org/research/ichthyology/catalog/SpeciesByFamily.asp [Electronic version last accessed 13.03.2017]
- Fontanele O (1948) Contribução para o conhemicento da biologia do Pirarucu, “Arapaima gigas” (Cuvier), em cativeiro (Actinopterygii, Osteoglossidae). Revista Brasileira de Biologia 8: 445–459.
- Friese UE (1980) Aquarium Fish. Tropical Fish Hobbyist Publication, 93 pp.
- Glenn CL, Williams RRG (1976) Fecundity of Mooneye, Hiodon tergisus in the Assiniboine River. Canadian Journal of Zoology, 1976, 54(2): 156–161. https://doi.org/10.1139/z76-016
- Greenwood PH (1963) The swimbladder in African Notopteridae (Pisces) and its bearing on the taxonomy of the family. Bulletin of the British Museum (Natural History) 11: 377–412. https://doi.org/10.5962/bhl.part.4720
- Greenwood PH (1973) Interrelationships of Osteoglossomorphs. In: Greenwood PH, Miles RS, Patterson C (Eds) Interrelationships of fishes.Academic Press, London, 307–332.
- Greenwood PH, Rosen DE, Weitzman SH, Myers GS (1966) Phyletic studies of teleostean fishes, with a provisional classification of living forms. Bulletin of the American Museum of Natural History 131: 339–456.
- Hamor T, Garside ET (1979) Hourly and total oxygen consumption by ova of Atlantic Salmon, Salmo salar L. in various combinations of temperature and dissolved oxygen. Canadian Journal of Zoology 57: 1196–1200. https://doi.org/10.1139/z79-152
- Haniffa MA, Raj AJA, Nagaraja M, Perumalsamy P, Seetharaman S, Singh PS (2004) Natural breeding in captivity – A possibility for conservation of threatened freshwater Featherback Notopterus notopterus. Aquaculture Asia 1, Genes and Fish 9(1): 36–38.
- Heming TA (1982) Effects of temperature on utilization of yolk by Chinook Salmon (Oncorhyncus tshawytscha) eggs and alevins. Canadian Journal of Fisheries and Aquatic Science 39: 184–190. https://doi.org/10.1139/f82-021
- Hilton EJ (2003) Comparative osteology and phylogenetic systematics of fossil and living Bony-tongue fishes (Actinopterygii, Teleostei, Osteoglossomorpha). Zoological Journal of the Linnean Society 137: 1–100. https://doi.org/10.1046/j.1096-3642.2003.00032.x
- Hossain QZ (1999) Some observations on breeding and fry rearing of “Chital” (Notopterus chitala Ham.) in Bangladesh. Fishing Chimes 19: 13–16.
- Hubbs CL (1926) The structural consequences of modifications of the developmental rate in fishes, considered in reference to certain problems of evolution. American Naturalist 60: 57–81. https://doi.org/10.1086/280071
- Johnson GH (1951) An investigation of Mooneye (Hiodon tergisus). Technical Sessions of the Research Council of Ontario 5, Abstract 16.
- Kirschbaum F (1975) Environmental factors control the periodical reproduction of tropical electric fish. Experentia 31: 1159–1160. https://doi.org/10.1007/BF02326767
- Kirschbaum F (1979) Reproduction of the weakly electric fish Eigenmannia virescens (Rhamphichthyidae, Teleostei) in captivity. I. Control of gonadal recrudescence and regression by environmental factors. Behaviour Ecology Sociobiology 4: 331–335. https://doi.org/10.1007/BF00303241
- Kirschbaum F (1987) Reproduction and development of the weakly electric fish Pollimyrus isidori (Mormyridae, Teleostei) in captivity. Environmental Biology of Fishes 20: 11–31. https://doi.org/10.1007/BF00002023
- Kirschbaum F (1994) Reproduction and development in Mormyriform and Gymnotiform fishes. In: Moller P (Ed.) Electric Fishes.History and Behaviour. Chapmann and Hall, London, 267–301.
- Kirschbaum F (1995) Vergleichende experimentelle Daten zur zyklischen Fortpflanzung tropischer Süßwasserfische. In Greven H, Riehl R (Eds) Fortpflanzungsbiologie der Aquarienfische. Birgit Schmettkamp Verlag, Bornheim, 69–80.
- Kirschbaum F (2006) Erstmalige Zucht eines Vertreters der Nilhechtgattung Petrocephalus (P. soudanensis) induziert durch Imitation von Hochwasserbedingungen. In: Greven H, Riehl R (Eds) Biologie der Aquarienfische.Tetra-Verlag, 65–71.
- Kirschbaum F, Leyendecker U, Nyonge B, Schulz C, Weitkamp H, Diedhiou S, Thomas M, Schugardt C (2008) Environmental control of cyclical reproduction of tropical freshwater fish: evidence from comparative experimental data. Cybium 32(2): 294–296.
- Kirschbaum F, Schugardt C (1995) Vergleichende Daten zur Fortpflanzungsbiologie von zwei Nilhecht-Arten (Mormyridae). In Greven H, Riehl R (Eds) Fortpflanzungsbiologie der Aquarienfische. Birgit Schmettkamp Verlag, Bornheim, 81–90.
- Kirschbaum F, Schugardt C (2002) Reproductive strategies and developmental aspects in Mormyrid and Gymnotiform Fishes. Journal of Physiology 96: 557–566. https://doi.org/10.1016/s0928-4257(03)00011-1
- Kunz YW (2004) Developmental biology of Teleostei. Fish and Fisheries Series. Springer, 636 pp. https://doi.org/10.1007/978-1-4020-2997-4
- Lake JS, Midgley SH (1970) Australian Osteoglossidae (Teleostei). Australian Journal of Science 32: 442–443.
- Li GQ, Wilson MVH (1996) Phylogeny of Osteoglossomorpha. In Stiassny MLJ, Parenti LR, Johnson GD (Eds) Interrelationships of fishes. Academic Press, San Diego, 163–174.
- Lüling KH (1964) Zur Biologie und Ökologie von Arapaima gigas (Pisces, Osteoglossidae). Zeitschrift für Morphologie und Ökologie der Tiere 54: 436–530. https://doi.org/10.1007/BF00395889
- Merrick JR, Schmida GE (1984) Australian freshwater fishes. Biology and Management. Griffin Press Ltd., South Australia, 409 pp.
- Mookerjee HK, Mazumdar SR (1946) On the life history of Notopterus notopterus (Pallas). Journal of the Department of Sciences, Calcutta University 2: 88–100.
- Moreau J (1974) Premières observations ecologiques sur la reproduction d´Heterotis niloticus (Osteoglossidae). Annales d’Hydrobiologie 5: 1–13.
- Mustafa G, Ahmed ATA (1979) Food of Notopterus notopterus (Pallas) (Notopteridae: Clupeiformes). Bangladesh Journal of Zoology 1: 7–14.
- Nawar G (1959) Observations on breeding of six members of the Nile Mormyridae. Annals and Magazine of NaturalHistory, Ser. 13, 2: 493–504. https://doi.org/10.1080/00222935908650882
- Nelson JS (2006) Fishes of the world.4th Edition. John Wiley & Sons, 601 pp.
- Nguyen MDL (2011) Reproduction and development of mormyrid fish of the Paramormyrops magnostipes-complex. Master Thesis. Humboldt Universität zu Berlin.
- Nyonje BM (2006) Experimental studies on cyclical reproduction of tropical African freshwater fishes. Dissertation. Humboldt Universität zu Berlin.
- Nysten M (1962) Étude anatomique des rapports de la vessie aerienne avec l`axe vertebral chez Pantodon buchholzi Peters. Annales de Musée Royal d’Afrique Central, Tervuren, Ser. 8°, Sciences Zoologiques 108: 185–225.
- Ong KY (1965) Spawning Notopterus afer. Tropical Fish Hobbyist 14(2): 24, 27, 30.
- Peňáz M, Prokes MJ, Hamackova J (1983) Early development of the Carp, Cyprinus carpio. Acta Societatis Zoologicae Bohemicae, Brno, 17: 1–39.
- Pinxteren MCA van (1974) Gelungene Zucht mit einem Messerfisch, Notopterus spec. Aquarien und Terrarien Zeitschrift 37: 364–369.
- Radheshyam N, Sarangi N (2005) Breeding and egg incubation of Notopterus chitala (Hamilton) in captivity. Journal of the Inland Fisheries Society of India 37: 8–14.
- Riehl R (1991) Structure of oocytes and egg envelopes in oviparous teleosts. An overview. Acta Biologica Benrodis 3: 27–65.
- Riehl R, Kokoscha M (1993) A unique surface pattern and micropylar apparatus in the eggs of Luciocephalus sp. (Perciformes, Luciocephalidae). Journal of Aquaculture and Aquatic Science 6: 1–6. https://doi.org/10.1111/j.1095-8649.1993.tb00444.x
- Riehl R, Patzner RA (1992) The eggs of native fishes. 3. Pike – Esox lucius L. 1758. Acta Biologica Benrodis 4: 135–139.
- Roberts TR (1992) Systematic revision of the old world freshwater fish family Notopteridae. Ichthyological Explorations of Freshwaters 2(4): 361–383.
- Sakar UK, Deepak PK, Negy RS, Singh S, Kapoor D (2006) Captive breeding of endangered fish Chitala chitala (Hamilton-Buchanan) for species conservation and sustainable utilization. - Marine, Freshwater, and Wetlands Biodiversity Conservation. Topics in Biodiversity and Conservation. Volume 4, 2006, 211–221. https://doi.org/10.1007/978-1-4020-5734-2_15
- Schugardt C, Kirschbaum F (2004) Control of gonadal maturation and regression by experimental variation of environmental factors in the mormyrid fish, Mormyrus rume proboscirostris. Environmental Biology of Fishes 70: 227–233. https://doi.org/10.1023/B:EBFI.0000033340.49266.f3
- Scott DBC (1973) The reproductive cycle of Mormyrus kannume Forsk. (Osteoglossomorpha, Mormyriformes) in Lake Victoria Uganda. Journal of Fish Biology 6: 447–454. https://doi.org/10.1111/j.1095-8649.1974.tb04560.x
- Siraad HA (1999) Kweek van Xenomystus nigri, de Afrikaanese mesvis. Het Aquarium 69(3): 90–92.
- Smith HM (1933) Contributions to the ichthyology of Siam. VII. The Featherback fish Notopterus chitala in Siam with notes on its egg-laying and young. Journal of the Siam Society of Natural History, Supplement 9: 245–258.
- Snyder DE, Douglas SC (1978) Description and identification of Mooneye, Hiodon tergisus, protolarvae. Transactions of the American Fisheries Society 170: 590–594. https://doi.org/10.1577/1548-8659(1978)107<590:DAIOMH>2.0.CO;2
- Southwell T, Prashad B (1919) Notes from the Bengal fisheries laboratory, No. 6. Records of the Indian Museum 26: 215–240.
- Srivastava SM, Gopalakrishnan A, Singh SP, Pandey AK (2012) Embryonic and larval development of threatened Bronze Featherback, Notopterus notopterus (Pallas). Journal of Experimental Zoology of India 15(2): 425–430.
- Stacey NE (1989) Control of the timing of ovulation by exogeneous and endogeneous factors. In Potts GW, Wootton RJ (Eds) Fish Reproduction. Strategies and Tactics. Academic Press, London, 410 pp.
- Trittelvitz W (1986) Beobachtungen bei der Zucht von Xenomystus nigri. Aquarien und Terrarienzeitschrift 39: 574.
- Wallace RA, Selman K (1981) Cellular and dynamic aspects of oocyte growth in teleosts. American Zoologist 21: 325–343. https://doi.org/10.1093/icb/21.2.325
- Wallus R (1990) Family Hiodontidae. In: Wallus R, Simon TP, Yaeger BL (Eds) Reproductive biology and early life history of fishes of the Ohio River Drainage.Vol. 1 Acipenseridae through Esocidae. Tennessee Valley Authority, Chatanooga, 153–166.
- Weitkamp H (2005) Untersuchungen zur Reproduktionsbiologie von Notopterus notopterus (Pallas, 1769). Bachelor-Arbeit im Studiengang Agrarwissenschaften. Humboldt-Universität zu Berlin.