Research Article |
|
Corresponding author: U. Kutschera ( kut@uni-kassel.de ) Academic editor: Matthias Glaubrecht
© 2014 U. Kutschera, Joy Elliott.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Kutschera U, Elliott J (2014) The European medicinal leech Hirudo medicinalis L.: Morphology and occurrence of an endangered species. Zoosystematics and Evolution 90(2): 271-280. https://doi.org/10.3897/zse.90.8715
|
Abstract
Although the European medicinal leech (Hirudo medicinalis L. 1758) is one of the best-known members of the Hirudinea due to its use in phlebotomy, this species has been confused with the Mediterranean taxon H. verbana
Key Words
Endangered species, evolution, Hirudo medicinalis , medicinal leeches, taxonomy
Introduction
Among the currently ca. 14 000 accepted species of Annelida (segmented worms) found worldwide in freshwater, marine and terrestrial ecosystems,
During subsequent decades, notably when the use of leeches in phlebotomy (bloodletting) became very popular throughout Europe (ca. 1850), numerous “varieties” of “H. medicinalis” were distinguished by naturalists as well as practitioners (
In his classic monograph on leeches,
In the present article, we describe the morphology of juvenile and adult H. medicinalis-individuals, add information on its evolutionary distance to its sister taxon H. verbana, and summarize observations on the behaviour, ecology and distribution of this endangered species.
Materials and methods
Adult and juvenile European medicinal leeches (H. medicinalis) (plus cocoons) were obtained from undisturbed habitats of eastern Germany (Elliott and
Results
Morphology of adult H. medicinalis-individuals
Leeches are animals with an organization akin to that of earthworms, but having certain modifications associated with a predatory or parasitic mode of life. The limitation of the number of body segments facilitates a greater degree of agility than would be the case if the body was as long as that of most earthworms. The segments are each subdivided into a number of annuli, five in the Hirudinidae. There is some disagreement about the relationship between annulation and segmentation (
The size of the suckers relative to the body varies according to the mode of life of the leech species and, in H. medicinalis, the anterior sucker is quite small. The buccal cavity is lined by muscular ridges surmounted by cuticular teeth, and the mouth is a wide aperture occupying the whole of the anterior sucker (Fig.
The clitellum is situated towards the anterior of the body (Fig.
Photograph of living adult specimens of the European medicinal leech (H. medicinalis
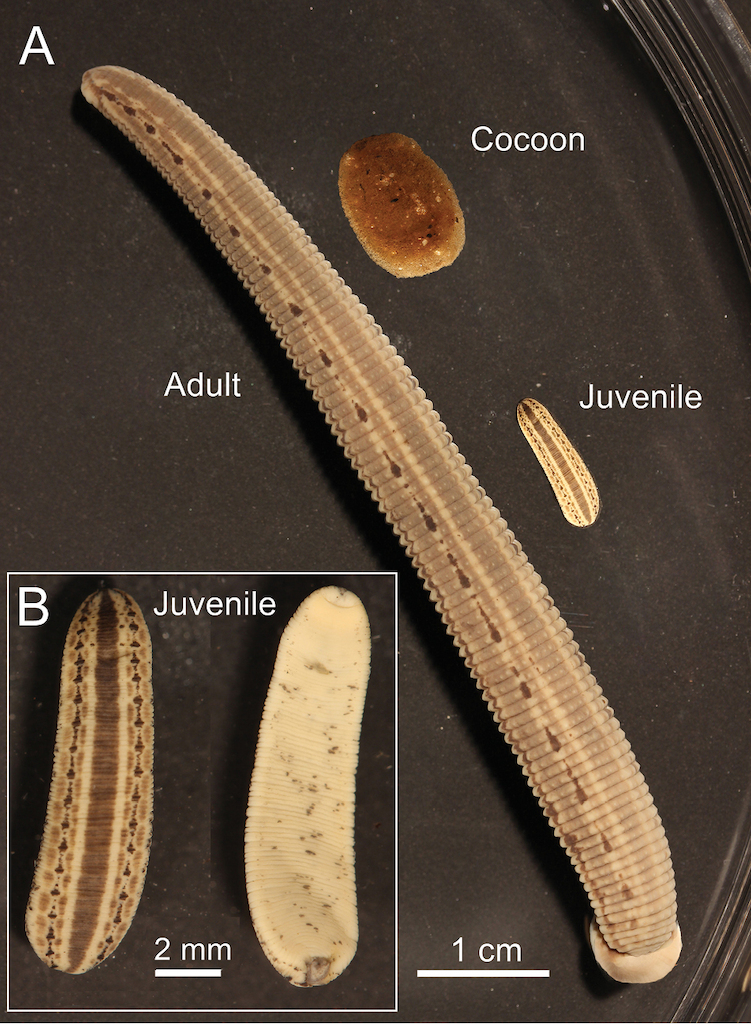
Cocoons and juvenile H. medicinalis
Mature medicinal leeches leave the water to deposit their cocoons in a moist place just above the water line on the shore or bank. The spongy cocoons (Fig.
The markings of the juveniles are very similar to those of the adults except there is less pigment on the ventral surface (Figs
Intact (A) and fragmented (B) posterior sucker of an adult alcohol-preserved H. medicinalis. The disk-shaped sucker is largely composed of muscle tissue containing numerous mitochondria. DNA-extractions for mt-sequence analysis (fragments of the gene CO-I) were performed from this part of the body that is not contaminated with the gut content of the blood-sucking annelid.
Behaviour of H. medicinalis vs. H. verbana and hyperparasitism
Living, adult individuals of H. medicinalis and its sister species H. verbana were maintained in aqua-terraria. Despite the fact that the species were clearly distinguishable based on their pigment patterns on both the dorsal and ventral sides of their body (Fig.
Laboratory experiments have also shown that when a medicinal leech is near a mammalian host, such as the skin of a human, it uses heat detection, the optimum response occurring at 33 to 40 °C (
However, other leech species will sometimes feed on H. medicinalis. Young Glossiphonia complanata that were co-cultivated with medicinal leeches frequently obtain their first meal by feeding on the body of H. medicinalis. In a quantitative study in a tarn (= pond) in Northwest England, H. medicinalis were found to be carrying all sizes of Helobdella stagnalis that were feeding on the host. The proboscis was inserted deep into the body wall of the host and the anterior portion of the body contracted regularly as fluid was extracted from the host, i.e., hyperparasitism was documented unequivocally. H. stagnalis did not kill its host or produce any obvious reactions. Similar observations were reported for H. verbana (
Phylogenetic analysis, divergence time and geographic distribution
In order to verify the taxonomic status of H. medicinalis from Germany (Figs
Based on CO-I-sequences acquired in our laboratory for H. verbana and other leech species (
Genetic distance between the type species of the Hirudinea, Hirudo medicinalis L. 1758, and other leeches, based on mitochondrial DNA-sequence data. The GenBank Accession Numbers for the mt-gene cytochrome c oxidase subunit I (CO-I) are added. AF = Africa, AS = Asia, EU = Europe, US = United States.
| Taxon | Locality | GenBank Acc.-No. CO-I | Identity (%) |
|---|---|---|---|
| Hirudo medicinalis | Sweden, EU | HQ333519 | 100 |
| Hirudo verbana | Turkey, EU/AS | EF125043 | 90.6 |
| Haemopis sanguisuga | Sweden, EU | AF462021 | 83.3 |
| Hirudinaria mallinensis | Malaysia, AS | AY425449 | 82.4 |
| Erpobdella octoculata | Germany, EU | AF003274 | 75.0 |
| Trocheta intermedia | Germany, EU | DQ009669 | 74.1 |
| Glossiphonia complanata | Germany, EU | AF003277 | 77.7 |
| Helobdella californica | California/US | HQ686307 | 73.5 |
| Malagabdella fallax | Madagascar, AF | EF125044 | 79.5 |
| Xerobdella lecomtei | Austria, EU | EF125040 | 76.0 |
Geographical distribution of Hirudo medicinalis and H. verbana, based on data published in 2012 (A). In the species H. verbana, a western (w) and an eastern (e) phylogroup has been identified. Occurrence of medicinal leeches in the nest of aquatic birds (B). The photograph shows adult, living specimens of H. medicinalis (with cocoon, see Inset) collected from a nest of a water bird (western marsh harrier, Circus aeruginosus) in Poland (adapted from
Discussion
The historical use, ecology, genetics and conservation of medicinal leeches was recently summarized (Elliott and
Large numbers of H. medicinalis were obtained from the wild in the 18th and early 19th centuries, and towards the end of this period, they were already scarce in many countries. This demand for medicinal leeches was not restricted to Europe. Hirudo medicinalis does not occur naturally in North America, and large numbers were imported from Europe into the United States in the 18th and 19th centuries. Several attempts were made to rear this species in the US, without positive results (Elliott and
H. verbana was first described from Lago Maggiore in Northeast Italy (Latin: Lacus Verbanus) by
Earlier reviews of the literature on the ecology of Hirudo medicinalis showed that there was surprisingly little quantitative information on medicinal leeches in the wild and most of the numerical values were from laboratory studies (
Six decades ago, laboratory studies showed that the preferred temperature of H. medicinalis in a gradient of 7 to 43 °C was 21 °C (
A number of explanations have been proposed for the loss of many populations of H. medicinalis in Northern Europe, and these should all be considered in combination. Extensive over-collecting for blood-letting in the nineteenth century is frequently blamed, but used leeches were regularly discarded into the nearest pond or stream and thus may have enabled the survival of this species in the countryside. Contemporary collecting for experimental biology, medical use and pharmaceutical needs is probably a serious threat because the leeches are destroyed, often in large numbers (
A reduction in the availability of suitable vertebrate hosts is another possible reason for the decline in countries where troughs are now used instead of ponds for the watering of cattle and horses. Changes in land use not only caused the loss of ponds but also isolation of the remaining freshwater ecosystems, even to wild animals such as deer, and this may have contributed to a reduction in blood meals from this source. However, there are still many parts of Europe where wild animals such as deer are plentiful, and therefore the almost complete absence of H. medicinalis in these areas is not due to a lack of mammalian hosts.
Water temperature will also affect the growth of H. medicinalis. Fast-growing leeches that attained maturity after only 289 days were kept at a constant 20 °C (
Finally, we want to point out that, although the distinctive features between H. medicinalis and H. verbana are obvious (Fig.
Hence, despite the fact that the European medicinal leech is, in addition to the taxonomically diverse earthworm Lumbricus terrestris (
Life history variables and reproductive success (i.e., number of offspring per individual and life time) of H. medicinalis, cultivated under sub-optimal laboratory conditions (20 °C). The animals were subsisted on mammalian (bovine) blood (n = 30) (adapted from
| Parameter | Range | Mean (± SE) |
|---|---|---|
| Time (years) from hatching to death | 1.3–2.3 | 2±0.1 |
| Cocoons produced/individual | 2–41 | 12±5 |
| Hatchlings/cocoon | 0–14 | 4±1 |
| Offspring produced/individual | 13–97 | 45±13 |
Acknowledgements
We thank Mr. M. Aurich (Biebertaler Blutegelzucht, Germany) for the provision of living medicinal leeches and Mr. U. Manzke for the photograph depicted in Fig.
References
- Benson DA, Cavanaugh M, Clark K, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW (2013) GenBank. Nucleic Acids Research 41: 36–42. doi: 10.1093/nar/gks1195
- Benecke H-G (2009) Erdkröten (Bufo bufo) werden von Egeln (Hirudinea) attackiert. Artenschutzreport 23: 63–64.
- Buczyńsky P, Tończyk G, Bielecki A, Cichocka JM, Kitowski I, Grzywaczewski G, Krawczyk R, Nieoczym M, Jabłońska A, Pakulnicka J, Buczyńska E (2014) Occurrence of the medicinal leech (Hirudo medicinalis) in birds‘ nests. Biologia 69: 484–488. doi: 10.2478/s11756-014-0329-0
- Carena H (1820) Monographie du genre Hirudo ou description des espéces de sangsues qui se trouvent ou qui sont en usage en piémont, avec des observations sur la génération, et sur d’autres points de l’histoire naturelle de quelques unes de ces espéces. Memorie della Reale Accademia delle Scienze die Torino 25: 273–316.
- Davies RW, McLoughlin NJ (1996) The effects of feeding regime on the growth and reproduction of the medicinal leech Hirudo medicinalis. Freshwater Biology 36: 563–568. doi: 10.1046/j.1365-2427.1996.00121.x
- De Salle R, Egan MG, Siddall ME (2005) The unholy trinity: taxonomy, species delimitation and DNA barcoding. Philosophical Transactions of the Royal Society B 360: 1905–1916. doi: 10.1098/rstb.2005.1722
- Dickinson MH, Lent CM (1984) Feeding behaviour of the medicinal leech, Hirudo medicinalis L. Journal of Comparative Physiology A 154: 449–455. doi: 10.1007/BF00610160
- Elliott EJ (1986) Chemosensory stimuli in feeding behavior of the leech Hirudo medicinalis. Journal of Comparative Physiology 159: 391–401. doi: 10.1007/BF00603984
- Elliott JM (2008) Population size, weight distribution and food in a persistent population of the rare medicinal leech, Hirudo medicinalis. Freshwater Biology 53: 1502–1512. doi: 10.1111/j.1365-2427.2008.01978.x
- Elliott JM, Dobson M (2014) Freshwater Leeches (Hirudinea) of Britain and Ireland; Keys and a Review of their Ecology. Freshwater Biological Association, Scientific Publication No. 69. Freshwater Biological Association, Ambleside, 90 pp.
- Elliott JM, Kutschera U (2011) Medicinal leeches: Historical use, ecology, genetics and conservation. Freshwater Reviews 4: 21–41. doi: 10.1608/FRJ-4.1.417
- Elliott JM, Mann KH (1979) A key to the British Freshwater Leeches with Notes on their Life Cycles and Ecology. Freshwater Biological Association, Scientific Publication No. 40. Freshwater Biological Association, Ambleside, 72 pp.
- Elliott JM, Tullett PA (1984) The status of the medicinal leech Hirudo medicinalis in Europe and especially in the British Isles. Biological Conservation 29: 15–26. doi: 10.1016/0006-3207(84)90011-9
- Elliott JM, Tullett PA (1986) The effects of temperature, atmospheric pressure and season on the swimming activity of the medicinal leech, Hirudo medicinalis (Hirudinea; Hirudinidae), in a Lake District tarn. Freshwater Biology 16: 405–415. doi: 10.1111/j.1365-2427.1986.tb00981.x
- Elliott JM, Tullett PA (1992) The medicinal leech. Biologist 39: 153–158.
- Friesen WO (1981) Physiology of water motion detection in the medicinal leech. Journal of Experimental Biology 92: 255–275.
- Grosser C (2004) Rote Liste der Egel (Hirudinidae) des Landes Sachsen-Anhalt. Berichte des Landesamtes für Umweltschutz Sachsen-Anhalt 39: 161–164.
- Hechtel FOP, Sawyer RT (2002) Toward a taxonomic revision of the medicinal leech Hirudo medicinalis Linnaeus, 1758 (Hirudinea: Hirudinidae): re-description of Hirudo troctina Johnston, 1816 from North Africa. Journal of Natural History 36: 1269–1289. doi: 10.1080/00222930110048945
- Herter K (1936) Physiologie der Hirudineen. In: Klassen HGB, des Tierreichs O (Eds) Buch Hirudineen – Teil 2.Akademische Verlagsgesellschaft, Leipzig, 123–320.
- Herter K (1937) Ökologie der Hirudineen. In: Klassen HGB, des Tierreichs O (Eds) Buch Hirudineen – Teil 2.Akademische Verlagsgesellschaft, Leipzig, 321–496.
- Herter K (1968) Der Medizinische Blutegel und seine Verwandten. A. Ziemsen Verlag, Wittenberg Lutherstadt, 199 pp.
- Hoffman J (1960) Archives de la Section des Sciences Naturelle, Physiques et Mathematiques de l’Institut Grand-Ducal de Luxembourg 27: 285–291.
- James SW, Porco D, Decaëns T, Richard B, Rougerie R, Erséus C (2010) DNA barcoding reveals cryptic diversity in Lumbricus terrestris L. 1758 (Clitellata): Resurrection of L. herculeus (Savigny, 1826). PloS ONE 5/12: e15629. doi: 10.1371/journal.pone.0015629
- Jueg U (2009) Der Medizinische Blutegel (Hirudo medicinalis L. 1758) in Mecklenburg-Vorpommern. Mitteilungen der NGM 9: 3–14.
- Kaiser F (1954) Beiträge zur Bewegungsphysiologie der Hirudineen. Zoologische Jahrbücher Systematik, Ökologie und Geographie der Tiere 65: 59–90.
- Kovalenko M, Utevsky SY (2012) Size structures and comparative phenology of syntopic populations of Hirudo verbana and Hirudo medicinalis in eastern Ukraine. Biologia 67: 934–938. doi: 10.2478/s11756-012-0089-7
- Kutschera U (2004) Species concepts: leeches versus bacteria. Lauterbornia 52: 171–175.
- Kutschera U (2006) The infamous bloodsuckers from Lacus Verbanus. Lauterbornia 56: 1–4.
- Kutschera U (2007) Leeches underline the need for Linnaean taxonomy. Nature 447: 775. doi: 10.1038/447775b
- Kutschera, U. (2010) A new leech species from Southern Germany, Trocheta intermedia nov. sp. (Hirudinea: Erpobdellidae). Lauterbornia 70: 1–9.
- Kutschera U (2011) The Golden Gate Leech Helobdella californica (Hirudinea: Glossiphoniidae): Occurrence and DNA-based taxonomy of a species restricted to San Francisco. International Review of Hydrobiology 96: 286–295. doi: 10.1002/iroh.201111311
- Kutschera U (2012a) The Hirudo medicinalis species complex. Naturwissenschaften 99: 433–434. doi: 10.1007/s00114-012-0906-4
- Kutschera U (2012b) Hilfreiche Blutsauger in der Medizin und ihre Systematik. Biologie in unserer Zeit 42(6): 352–353. doi: 10.1002/biuz.201290093
- Kutschera U, Langguth H, Kuo D-H, Weisblat DA, Shankland M (2013) Description of a new leech species from North America, Helobdella austinensis n. sp. (Hirudinea: Glossiphoniidae), with observations on its feeding behaviour. Zoosystematics and Evolution 89: 239–246. doi: 10.1002/zoos.201300010
- Kutschera U, Pfeiffer I, Ebermann E (2007) The European land leech: biology and DNA-based taxonomy of a rare species that is threatened by climate warming. Naturwissenschaften 94: 967–974. doi: 10.1007/s00114-007-0278-3
- Kutschera U, Roth M (2005) Cannibalism in a population of the medicinal leech (Hirudo medicinalis L.). Biology Bulletin 32: 626–628. doi: 10.1007/s10525-005-0154-7
- Kutschera U, Roth M (2006) Cocoon deposition and cluster formation in populations of the leech Hirudo verbana (Hirudinea: Hirudinidae). Lauterbornia 56: 5–8.
- Kutschera U, Roth M, Ewert JP (2010) Feeding on bufoid toads and occurrence of hyperparasitism in a population of the medicinal leech (Hirudo verbana Carena 1820). Research Journal of Fisheries and Hydrobiology 5: 9–13.
- Kvist S, Oceguera-Figueroa A, Siddall ME, Erséus C (2010) Barcoding, types and the Hirudo files: Using information content to critically evaluate the identity of DNA barcodes. Mitochondrial DNA 21: 198–205. doi: 10.3109/19401736.2010.529905
- Lamarck J-B (1818) Histoire Naturelle des Animaux sans Vertèbres. Vol. 5. Baillière, Paris, 612 pp.
- Linnaeus C (1758) Systema naturae – Regnum animale, Editio decima. Holmiae, Laurenti Salvii, 824 pp.
- Mann KH (1962) Leeches (Hirudinea) – Their Structure, Physiology, Ecology and Embryology. Pergamon Press, London, 201 pp.
- Manzke U, Winkler C (2012) Amphibien als Wirt des Medizinischen Blutegels (Hirudo medicinalis)–Literaturauswertung und Aufruf zur Mitarbeit. RANA 13: 41–53.
- Michalsen A, Roth M (Hg.) (2006) Blutegeltherapie. Karl F. Haug Verlag, Stuttgart, 145 pp.
- Nesemann H, Neubert E (1999) Annelida, Clitellata: Branchiobdellidae, Acanthobdellea, Hirudinea. Spektrum, Heidelberg, 178 pp.
- Petrauskiené L, Utevska O, Utevsky S (2011) Reproductive biology and ecological strategies of three species of medicinal leeches (genus Hirudo). Journal of Natural History 45: 737–747. doi: 10.1080/00222933.2010.535918
- Phillips AJ, Siddall ME (2009) Polyparaphyly of Hirudinidae: many lineages of medicinal leeches. BMC Evolutionary Biology 9: 246–257. doi: 10.1186/1471-2148-9-246
- Reece JB, Urry LA, Cain ML (2011) Campbell Biology. 7th Edition. Pearson-Benjamin Cummings, San Francisco, 1464 pp.
- Russel PJ, Hertz PE, McMillan B (2014) Biology: The Dynamic Science. 3th Edition. Brooks/Cole Cenage Learning, Australia, 1306 pp.
- Sartor C, Bornet C, Guinard D, Fournier P-E (2013) Transmission of Aeromonas hydrophilia by leeches. Lancet 381: 1686. doi: 10.1016/S0140-6736(13)60316-5
- Sawyer RT (1986) Leech Biology and Behaviour. Oxford University Press, Oxford, 1065 pp.
- Sawyer RT (2013a) Re-assessment of the medicinal leech Hirudo medicinalis Linnaeus, 1758, in Ireland. Zoosystema 35: 113–123. doi: 10.5252/z2013n1a9
- Sawyer RT (2013b) History of the leech trade in Ireland, 1750–1915: Microcosm of a global commodity. Medical History 57: 420–441. doi: 10.1017/mdh.2013.21
- Shain DH (Ed.) (2009) Annelids in Modern Biology. John Wiley & Sons, Inc, New York, 358 pp.
- Siddall ME, Trontelj P, Utevsky SY, Nkamany M, Macdonald KS (2007) Diverse molecular data demonstrate that commercially available medicinal leeches are not Hirudo medicinalis. Proceedings of the Royal Society B 274: 1481–1487. doi: 10.1098/rspb.2007.0248
- Starr C, Evers CA, Starr L (2011) Biology. Concepts and Applications: Without Physiology. 8th Edition. Brooks/Cole Cenage Learning, Australia, 502 pp.
- Trontelj P, Sotler M, Verovnik R (2004) Genetic differentiation between two species of the medicinal leech, Hirudo medicinalis and the neglected H. verbana, based on random-amplified polymorphic DNA. Parasitology Research 94: 118–124.
- Trontelj P, Utevsky SY (2005) Celebrity with a neglected taxonomy: molecular systematics of the medicinal leech (genus Hirudo). Molecular Phylogenetics and Evolution 34: 616–624. doi: 10.1016/j.ympev.2004.10.012
- Trontelj P, Utevsky SY (2012) Phylogeny and phylogeography of medicinal leeches (genus Hirudo): Fast dispersal and shallow genetic structure. Molecular Phylogenetics and Evolution 63: 475–485. doi: 10.1016/j.ympev.2012.01.022
- Utevsky SY, Trontelj P (2005) A new species of the medicinal leech (Oligochaeta, Hirudinida, Hirudo) from Transcaucasia and an identification key for the genus Hirudo. Parasitology Research 98: 61–66. doi: 10.1007/s00436-005-0017-7
- Utevsky SY, Zinenko AI, Atemasov AA, Huseynov MA, Utevska OM, Utevsky AY (2008) New information on the distribution of the medicinal leech (genus Hirudo) in the Iberian Peninsula, the Caucasus and Central Asia. Lauterbornia 65: 119–130.
- Utevsky S, Zagmajster M, Atemasov A, Zinenko O, Utevska O, Utevsky A, Trontelj P (2010) Distribution and status of medicinal leeches (genus Hirudo) in the Western Palaeartic: anthropogenic, ecological, or historical effects? Aquatic Conservation 20: 198–210. doi: 10.1002/aqc.1071
- Vinarski MV (2014) The birth of malacology: When and how? Zoosystematics and Evolution 90: 1–5. doi: 10.3897/zse.90.7008
- Wells S, Coombes W (1987) The status and trade in the medicinal leech. Traffic Bulletin 8: 64–69.
- Wells SM, Elliott JM, Tullett PA (1984) Status of the medicinal leech Hirudo medicinalis. Biological Conservation 30: 379–380. doi: 10.1016/0006-3207(84)90057-0
- Westendorff M, Kalettka T, Jueg U (2008) Occurrence of leeches (Hirudinea) in different types of water bodies in northeast Germany (Brandenburg). Lauterbornia 65: 153–162.
- Wilkin PJ, Scofield AM (1990) The use of a serological technique to examine host selection in a natural population of the medicinal leech, Hirudo medicinalis. Freshwater Biology 23: 165–169. doi: 10.1111/j.1365-2427.1990.tb00261.x
- Wilkin PJ, Scofield AM (1991a) The structure of a natural population of the medicinal leech, Hirudo medicinalis, at Dungeness, Kent. Freshwater Biology 25: 539–546. doi: 10.1111/j.1365-2427.1991.tb01397.x
- Wilkin PJ, Scofield AM (1991b) Growth of the medicinal leech, Hirudo medicinalis, under natural and laboratory conditions. Freshwater Biology 25: 547–553. doi: 10.1111/j.1365-2427.1991.tb01398.x
- Winkler C, Manzke U (2014) Funde der Blutegelarten Hirudo medicinalis und Hirudo verbana in Norddeutschland unter Berücksichtigung von Amphibien als Wirtsorganismen–Ergebnisse eines Aufrufs in der RANA 13. RANA 15: 60–66.
- Wirchansky BA, Shain DH (2010) A new species of Haemopis (Annelida: Hirudinea): Evolution of North American terrestrial leeches. Molecular Phylogenetics and Evolution 54: 226–234. doi: 10.1016/j.ympev.2009.07.039
- Young SR, Dedwylder RD, Friesen WO (1981) Responses of the medicinal leech to water waves. Journal of Comparative Physiology A 144: 111–116. doi: 10.1007/BF00612804