Research Article |
|
Corresponding author: Salinee Khachonpisitsak ( salineek@go.buu.ac.th ) Academic editor: Michael Ohl
© 2017 Ashitapol Pochai, Sutin Kingtong, Woranop Sukparangsi, Salinee Khachonpisitsak.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Pochai A, Kingtong S, Sukparangsi W, Khachonpisitsak S (2017) The diversity of acorn barnacles (Cirripedia, Balanomorpha) across Thailand’s coasts: The Andaman Sea and the Gulf of Thailand. Zoosystematics and Evolution 93(1): 13-34. https://doi.org/10.3897/zse.93.10769
|
Abstract
The acorn barnacle is a sessile crustacean, inhabiting the intertidal areas of tropical and temperate regions worldwide. According to current practices on Cirripedia morphology, shell, opercular valves, and arthropodal characters including cirri and mouthparts are used as a tool for taxonomic classification, and using these characteristics the present study aimed to provide better resolution for the barnacle diversity and geographical distribution within coastlines of Thailand: the Andaman Sea and the Gulf of Thailand. A total of ten species belonging to three families (Chthamalidae, Tetraclitidae, and Balanidae) were identified in this study. Subsequently, five species were newly recorded for the first time from Thailand’s coasts: Newmanella spinosus Chan & Cheang, 2016, Euraphia hembeli Conrad, 1837, Euraphia depressa (Poli, 1795), Tetraclita kuroshioensis Chan, Tsang & Chu, 2007, and Tetraclita singaporensis Chan, Tsang & Chu, 2007. The others, already mentioned in previous records, include: Tetraclita squamosa (Bruguière, 1789), Chthamalus malayensis Pilsbry, 1916, Amphibalanus amphitrite (Darwin, 1854), Amphibalanus reticulatus (Utinomi, 1967), and Megabalanus tintinnabulum (Linnaeus, 1758). Interestingly, acorn barnacles along the Andaman Sea occur abundantly, and are much higher in number of species (up to 8 species) than those found in the Gulf of Thailand’s coast (up to 6 species). This biased trend of species’ preferences is possibly due to the differences in oceanographic nature between two coastlines and the history of barnacle colonization.
Key Words
acorn barnacle, Cirripedia , Balanomorpha , shell morphology, opercular valve, distribution, Thailand
Introduction
Acorn barnacles, a member of marine crustaceans, inhabit a diverse array of substrates (e.g. calcareous rock or limestone, mollusk shells, corals, sponges, mangrove roots, turtle shells, and whale skins) along intertidal zones of temperate and tropical coastlines worldwide, as sessile form throughout their adulthood (
Material and methods
Study sites
Acorn barnacles were collected from the rocky coastal areas of two distinct geographic regions of Thailand: the Andaman Sea and the Gulf of Thailand, during May 2015-July 2016.
The Andaman Sea located in the eastern part of the Indian Ocean is bordered by the coastlines of Myanmar, Thailand, Malaysia, Indonesia and India. In the Andaman Sea, the tide is semidiurnal. Its water temperature and salinity range 25.9–30.4 °C and 29–33 ppt, respectively (
The Gulf of Thailand, a semi-enclosed sea, is bordered by the coastlines of Vietnam, Cambodia, Thailand, and Malaysia with a connection to the South China Sea in the south. In the Gulf of Thailand, the tide is mixed diurnal. Its water temperature and salinity range 29–32 °C and 30–33 ppt, respectively (
A synopsis and illustration of all the sampling locations are given in Table
Map showing all sampling locations (A) and habitat characteristics (B) of acorn barnacles found along the coastlines of the Andaman Sea and the Gulf of Thailand. See Table
| Locality | Habitat characteristics | Coordinates | |
|---|---|---|---|
| Andaman Sea coast | |||
| Ao Khoei | AK | Large boulders on sandy shores | 09°16’44.18”N 098°22’07.01”E |
| Na Tai | NT | Rocky shores | 08°14’15.39”N 098°16’51.22”E |
| Kalim | KL | Small to large rocks on sandy shores | 07°55’25.47”N 098°15’47.68”E |
| Ao Yon | AY | Rocky shores | 07°52’09.79”N 098°26’08.29”E |
| Panwa | PW | Large boulders on sandy shores | 07°48’05.09”N 098°24’28.80”E |
| Gulf of Thailand coast | |||
| Khao Sam Muk | KS | Rocky shores | 13°18’38.88”N 100°54’07.81”E |
| Si Racha | SR | Large boulders on sandy shores | 13°10’33.92”N 100°55’33.74”E |
| Ko Kham Yai | KK | Small rocks on sandy shores | 13°09’59.30”N 100°49’18.00”E |
| Ban Krut | BK | Rocky shores | 11°21’26.07”N 099°34’42.86”E |
| Hin Ngam | HN | Rocky shores | 09°00’00.68”N 099°55’09.45”E |
Sampling collection
The barnacles were collected from each station by surveying along rocky shores of an intertidal zone during both low and high tides. Whole acorn barnacle individuals were removed from the substratum and immediately preserved in ethyl alcohol (95% v/v) for further examination. All work was done under certified supervision of S.K. (Certificate from Institute of Animal for Scientific Purposes Development-IAD, Royal Thai Government: U1-03104-2559).
Morphology analysis
Samples were primarily identified based on their shell morphology using an Olympus SZ51 stereomicroscope and was photographed with digital camera. For better species identification in some families, arthropodal characters were observed. Soft bodies were removed from the shells and dissected. Cirri and mouthparts were mounted onto slides for light microscopy observation and imaging using digital camera. Taxonomic identification was performed using keys of
Results
Based on shell morphology, total ten species (6 genera) of acorn barnacles along the coastlines of Thailand in both the Andaman Sea and the Gulf of Thailand were identified and are categorized into three families: Chthamalidae (2 subfamilies: Chthamalinae and Euraphiinae), Tetraclitidae (2 subfamilies: Newmanellinae and Tetraclitinae), and Balanidae (2 subfamilies: Amphibalaninae and Megabalaninae). The descriptions of the identified barnacles are as follows:
Systematic taxonomy
Superorder Thoracica Darwin, 1854
Order Sessilia Lamarck, 1818
Suborder Balanomorpha Pilsbry, 1916
Superfamily Chthamaloidea Darwin, 1854
Family Chthamalidae Pilsbry, 1916
Subfamily Chthamalinae Darwin, 1854
Chthamalus
Type species
Chthamalus stellatus (Poli, 1791)
1 genus, 1 species recorded: Chthamalus malayensis Pilsbry, 1916.
Chthamalus malayensis
Chthamalus
malayensis
Pilsbry, 1916: 310–311;
Chthamalus
stellatus
:
Chthamalus
challenger
:
Chthamalus
antennatus
:
Non-type material examined
Andaman Sea: 3 specimens, Phang-nga province, Takua Thung district, Na Tai beach, 16.V.2015, A. Pochai (BUU16.CH.CM01-03). 2 specimens, Phuket province, Mueang Phuket district, Ao Yon beach, 15.VII.2015, A. Pochai (BUU16.CH.CM04-05). 1 specimen, Phuket province, Mueang Phuket district, Panwa beach, 16.VII.2015, S. Khachonpisitsak (BUU16.CH.CM06). 3 specimens, Phuket province, Katu district, Kalim beach, 15.VII.2015, A. Pochai (BUU16.CH.CM07-09).
Gulf of Thailand: 2 specimens, Chon Buri province, Ko Si Chang district, Ko Kham Yai beach, 05.VII.2015, S. Khachonpisitsak (BUU16.CH.CM10-11).
Description
Peduncle absent; body length 3–10 mm; base membranous. Shell elongated oval/shield-shaped, shell white to grey with 6 plates (1 carina, 2 carinal latus, 2 latus and 1 rostrum), carina bigger than rostrum, parietes symmetrical, calcareous and solid, radii solid, inner surface of parietes smooth and white-grey to pale-violet; orifice kite-shaped. Operculum plates symmetrical, articulation of opercular valves deep, scutum and tergum separable. Tergum smaller than scutum, tergum higher than wide, tergum with 4 distinct crests for lateral depressor muscles. Scutum elongated and triangular, adductor pit deep. Mandible with 4 teeth, lower margin pectinated, three large setae at the edge; cirri I with conical spines; cirri II with multi-cuspidate setae and basal guard.
Distribution
Chthamalus malayensis is widely distributed in the Indo-West Pacific region. It has been previously recorded in Taiwan, Thailand, China, Philippines, Vietnam, Malaysia, India and Australia (
Species list and distribution of acorn barnacles found in ten sampling sites along the coastlines of the Andaman Sea and the Gulf of Thailand. Abbreviations: +, presence; abs, absence. See Table
| Species | Sampling sites | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Andaman Sea | Gulf of Thailand | |||||||||
| AK | NT | KL | AY | PW | KS | SR | KK | BK | HN | |
| Chthamalus malayensis | abs | + | + | + | + | abs | abs | + | abs | abs |
| Euraphia depressa | abs | abs | abs | abs | abs | + | abs | abs | abs | abs |
| Euraphia hembeli | abs | + | abs | abs | abs | abs | abs | abs | abs | abs |
| Newmanella spinosus | abs | + | abs | abs | abs | abs | abs | abs | abs | abs |
| Tetraclita kuroshioensis | + | + | + | + | abs | abs | abs | + | + | abs |
| Tetraclita singaporensis | abs | + | abs | abs | abs | abs | abs | abs | abs | abs |
| Tetraclita squamosa | abs | abs | abs | abs | abs | abs | abs | abs | abs | + |
| Amphibalanus amphitrite | + | + | + | + | + | + | + | + | + | + |
| Amphibalanus reticulatus | abs | + | abs | abs | abs | + | + | + | abs | abs |
| Megabalanus tintinnabulum | abs | + | abs | abs | abs | abs | abs | abs | abs | abs |
| Total number of species | 2 | 8 | 3 | 3 | 2 | 3 | 2 | 4 | 2 | 2 |
Remarks
Chthamalus malayensis has usually 4 crests for lateral depressor muscles while Euraphia hembeli and Euraphia depressa contains distinct 10–12 crests at the tergum and 3 small crests, respectively. The size of C. malayensis ranges from 3–10 mm similar to E. depressa while that of Euraphia hembeli is much bigger (10–33 mm). In addition, C. malayensis differs from E. depressa in two main characters diagnosed in this study: shape of external shell and jointing pattern of tergum and scutum. The shape of the external shell of C. malayensis shows a distinct and rather uniform ribbed surface from the lower region to the apex; on the other hand, E. depressa exhibits smooth surface that is never ribbed. Secondly, marked articulation and sinous jointing of tergum and scutum can be clearly noticed in C. malayensis while E. depressa shows less articulation. However, these shell morphology is not reliable tool for species identification among Chthamalids; hence, we further investigate arthropodal characters. It is clear that Chthamalus has four teeth on the mandible while Euraphia has three teeth on the mandible. In addition, to further identify Chthamalus into the correct species, setae on cirri I and cirri II were observed. Our specimens of Chthamalids have conical spines on cirri I and multi-cuspidate setae with basal guard on cirri II (Figure
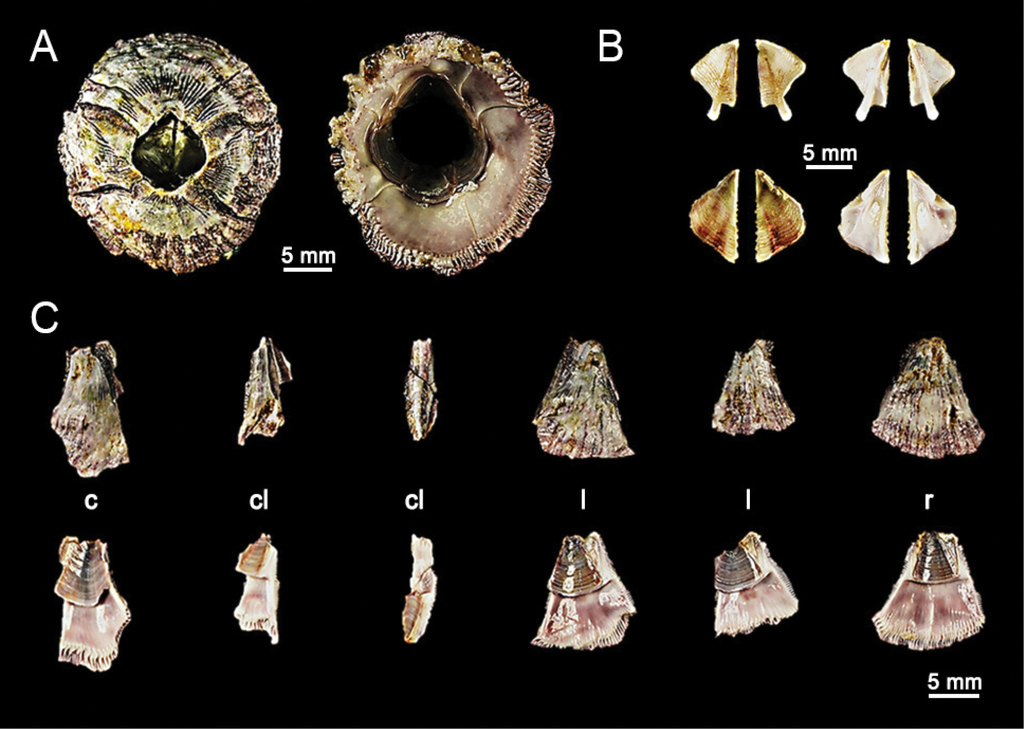
Chthamalus malayensis collected from Ka Lim beach, Phuket (BUU16.CH.CM07). A. Dorsal and ventral view of external shell, B. External (left panel) and internal (right panel) view of tergum (upper panel) and scutum (lower panel), C. External (upper panel) and internal (lower panel) view of shell plates, D–G. Light microscopy on mouthparts, D. Close up of cirri I showing conical spines(↑), E. Cirri II, F. Close up on cirri II showing multi-cuspidate setae with basal guard(↓), G. Mandible with four large teeth. D-G. Scale bars in µm. Abbreviations: c, carina; cl, carinal latus; l, latus; r, rostrum.
Moreover, C. malayensis distributes above the vertical zonation of Tetraclita population. The overlapping of habitats can be seen among these species and even C. malayensis were found to attach to Tetraclita at the overlapping regions of high shore and middle shore.
Subfamily Euraphiinae Newman & Ross, 1976
Euraphia
Type species
Euraphia hembeli Conrad, 1837
1 genus, 2 species recorded: Euraphia depressa (Poli, 1795) and Euraphia hembeli Conrad, 1837.
Euraphia depressa
Chthamalus depressus Poli, 1791
Chthamalus
stellatus
var.
depressus
:
Euraphia
depressa
:
Non-type material examined
Gulf of Thailand: 2 specimens, Chon Buri province, Mueang Chon Buri district, Khao Sam Muk beach, 05.VII.2016, W. Sukparangsi (BUU16.CM.ED01-02).
Description
Peduncle absent; body length 3–10 mm; base membranous. Shell light brown-yellowish brown with 6 plates (1 carina, 2 carinal latus, 2 latus and 1 rostrum), shell flatted and thin-walled; parietes symmetrical and solid, external surface of shell without ribbed, inner surface of parietes smooth and light brown and white with small horizontal striations around aperture, parietes separable, suture distinct and easily parted; orifice rhomboidal. Opercular plates symmetrical, tergum smaller than scutum, scutum and tergum separable jointing between tergum and scutum with slightly sinous. Scutum triangular with slightly curved basal margin, external surface with shallow and horizontal striations from occludent margin to tergal margin, occludent margin of scutum without teeth, tergal margin slightly sinous from interior view; tergum with 2–3 lateral depressor crests. Mandible with 3 teeth, lower margin pectinated with 8 setae, three large setae at the edge; labrum with obvious teeth; caudal appendage absent.
Distribution
In previous records, Euraphia depressa was found to inhabit along Mediteranean localities, including Spain (Punta Carnero, Punta de la Chullera, Malago, Salobrena and Calpe), France (Cap Bear, La Couronne, and Cassis), Italy (Pegli and Lido), Greece (Amnisso), the Black Sea and Suez canal (
Remarks
Euraphia depressa (Poli, 1795) was the reassigned name from Chthamalus depressus (Poli, 1795). According to
Euraphia depressa collected from Khao Sam Muk beach, Chon Buri (BUU16.CH.ED01). A. Dorsal and ventral view of external shell, B. External (left panel) and internal (right panel) view of tergum (upper panel) and scutum (lower panel), C. External (upper panel) and internal (lower panel) view of shell plates, D-G. Light microscopy on mouthparts, D. Labrum, E. Close up on the teeth of the labrum, F. Mandible with three large teeth, G. Close up on the pectinated lower margin of mandible. D–G. Scale bars in µm. Abbreviations: c, carina; cl, carinal latus; l, latus; r, rostrum.
Euraphia hembeli
Euraphia hembeli Conrad, 1837: 261.
Non-type material examined
Andaman Sea: 2 specimens, Phang-nga province, Takua Thung district, Na Tai beach, 16.V.2015, A. Pochai (BUU16.CH.EH01-02).
Description
Peduncle absent; base membranous; body length larger than Chthamalus and range from 10–30 mm. Shell brownish grey with 6 plates (1 carina, 2 carinal latus, 2 latus and 1 rostrum), carina bigger than rostrum, carinal latus bigger than latus. External surface of shell irregularly ribbed around basal margin, inner surface of parietes smooth and white with dark brown and pale violet horizontal striations around aperture. Parietes symmetrical, calcareous and solid, parietes separable, sutures coarsely serrate or with interlocking toothed structure. Orifice rhomboidal. Operculum plates symmetrical, tergum smaller than scutum, tergum and scutum separable. Scutum triangular, occludent margin of scutum with strong teeth. Tergum strongly marked with 10–12 lateral depressor crests, scutal margin strongly articulated.
Euraphia hembeli collected from Na Tai beach, Phang-nga (BUU16.CH.CH01). A. Dorsal and ventral view of external shell, B. External (left panel) and internal (right panel) view of tergum (upper panel) and scutum (lower panel), C. External (upper panel) and internal (lower panel) view of shell plates. Abbreviations: c, carina; cl, carinal latus; l, latus; r, rostrum.
Distribution
Barnacles in the genus Euraphia were recorded in several regions including West Africa, the Mediterranean, Hawaii and Southern Japan (
Distribution of acorn barnacles on different habitat types of intertidal zone (vertical zonation): low shores/sublittoral zone (LS), middle shores/littoral zone (MS), and high shores/supralittoral zone (HS).
| Scientific name | Habitat type | Settlement pattern on habitats | ||
|---|---|---|---|---|
| LS | MS | HS | ||
| Family Chthamalidae | ||||
| Chthamalus malayensis | + | Attached to rock platform, shell of Tetraclita spp. and other substrates | ||
| Euraphia depressa | + | Attached to sheltered sites of rock | ||
| Euraphia hembeli | + | + | Attached to rocky shore exposed to heavy wave action | |
| Family Tetraclitidae | ||||
| Newmanella spinosus | + | Attached to rocks on a wave exposed shore | ||
| Tetraclita kuroshioensis | + | Attached to rock platform and sheltered sites of rock | ||
| Tetraclita singaporensis | + | Attached to rock platform and sheltered sites of rock | ||
| Tetraclita squamosa | + | Attached to rock platform and sheltered sites of rock | ||
| Family Balanidae | ||||
| Amphibalanus amphitrite | + | + | + | Attached to rocks on a wave exposed shore, shell of oyster and Asian green mussel, offshore vessel and various substrates |
| Amphibalanus reticulatus | + | + | Attached to shell of Asian green mussel, oyster, ridged Venus clam and other substrates | |
| Megabalanus tintinnabulum | + | Attached to rocky shore exposed to heavy wave action | ||
Remarks
Based on the shell and opercular valve morphology (
Superfamily Tetraclitoidea Gruvel, 1903
Family Tetraclitidae Gruvel, 1903
Subfamily Newmanellinae Ross & Perreault, 1999
Newmanella
Type species
Newmanella radiata (Bruguiere, 1789)
1 genus, 1 species recorded: Newmanella spinosus Chan & Cheang, 2016.
Newmanella spinosus
Newmanella spinosus Chan & Cheang, 2016: 212–220, figs 9–15.
Non-type material examined
Andaman Sea: 4 specimens, Phang-nga province, Takua Thung district, Na Tai beach, 16.V.2015, A. Pochai (BUU16.TC.NS01-04).
Description
Peduncle absent; base calcareous. Shell greyish green, shell with 4 plates (1 carina, 2 latus, 1 rostrum); parietes low conical, 3–4 rows of irregular parietal tubes (parietes multiple tubiferous), radii board with horizontal striation and summit oblique. External surface with deep longitudinal/radiating lines from base to apex, internal surface of parietes smooth and white with greyish green striations close to operculum. Orifice pentagonal, diamond-shaped. External surface of operculum brownish grey, internal surface of operculum white. Scutum triangular, external surface of scutum with horizontal striations; tergum high and narrow, tergum with numerous depressor crests.
Newmanella spinosus collected from Na Tai beach, Phang-nga (BUU16.TC.NS01). A. Dorsal and ventral view of external shell, B. External (left panel) and internal (right panel) view of tergum (upper panel) and scutum (lower panel), C. External (upper panel) and internal (lower panel) view of shell plates. Abbreviations: c, carina; l, latus; r, rostrum.
Distribution
Newmanella spinosus was previously recorded from low intertidal to subtidal levels on rock shores along the coastlines of Taiwan and the Philippines and they were also collected from the surfaces of buoys used in fishing cages in the open sea (
Remarks
N. spinosus is morphologically similar to N. radiata, based on shell and scutum. The shell of N. spinosus is green while those of N. radiata is white. In addition, lateral scutal depressor muscle crest is shallow in the scutum of N. radiata, but deep in N. spinosus. The distribution of N. spinosus is around the North Pacific Ocean, from Okinawan Japan to Taiwan and the Philippines (
Subfamily Tetraclitinae Newman & Ross, 1976
Tetraclita
Type species
Tetraclita squamosa (Bruguiére, 1789)
1 genus, 3 species recorded: Tetraclita kuroshioensis Chan, Tsang & Chu, 2007, Tetraclita singaporensis Chan, Tsang & Chu, 2007 and Tetraclita squamosa (Bruguiere, 1789).
Tetraclita kuroshioensis
Tetraclita
squamosa
viridis
:
Tetraclita
squamosa
squamosal
:
Tetraclita
pacifica
Non-type material examined
Andaman Sea: 2 specimens, Phang-nga province, Khura Buri district, Ao Khoei beach, 30.VII.2015, A. Pochai (BUU16.TC.TK01-02). 3 specimens, Phang-nga province, Takua Thung district, Na Tai beach, 16.V.2015, A. Pochai (BUU16.TC.TK03-05). 2 specimens, Phuket province, Mueang Phuket district, Ao Yon beach, 15.VII.2015, A. Pochai (BUU16.TC.TK06-07). 3 specimens, Phuket province, Katu district, Kalim beach, 15.VII.2015, A. Pochai (BUU16.TC.TK08-10).
Gulf of Thailand: 3 specimens, Prachuap Khiri Khan province, Bang Saphan district, Ban Krut beach, 06.IX.2015, A. Pochai (BUU16.TC.TK11-13). 3 specimens, Chon Buri province, Ko Si Chang district, Ko Kham Yai beach, 05.VII.2015, S. Khachonpisitsak (BUU16.TC.TK14-16).
Description
Peduncle absent; base membranous; shell greyish black to purplish-grey with 4 plates (1 carina, 2 latus, 1 rostrum), parietes conical, plates inseparable, 7–8 rows of parietal tubes (parietes multiple tubiferous), external surface with mosaic scales pattern radiating randomly from base to apex, internal surface of parietes smooth and white with dark grey striations around aperture. External surface of operculum mixed grey and yellowish-light brown, internal surface of operculum greyish-dusky green. Scutum bigger than tergum, scutum triangular, external surface of scutum with horizontal striations, occludent margin of scutum with obvious shallow and rough teeth, short articular ridge-basal margin, angle between basal margin and tergal margin is quite perpendicular. Tergum higher than wide, basi-scutal angle 158°, tergum with broad spur, spur angle 30°. Mandible with 4 big teeth, 1st tooth smaller; maxillule not notched with 11 setae; labrum with 5 small teeth on each side; cirri I possessing serrulate setae.
Tetraclita kuroshioensis collected from (BUU16.TC.TK01) from Na Tai beach, Phang-nga. A. Dorsal and ventral view of external shell, B. External (left panel) and internal (right panel) view of tergum (upper panel) and scutum (lower panel), C–F. Light microscopy on mouthparts, C. Labrum, D. Close up on the teeth of the labrum, E. Mandible, F. Close up on the lower margin of mandible. C–F. Scale bars in µm.
Distribution
Tetraclita kuroshioensis is reassigned the name from Tetraclita squamosa which were collected from Taiwan, and Okinawa and Honsu of Japan, and Tetraclita pacifica. The distribution of this species occurs in broad area along north-west Pacific region (
Remark
Tetraclita kuroshioensis is quite similar to Tetraclita singaporensis in following characteristics: tergum without beak and with wide spur, scutum with short articular ridge-basal margin. However, angle between tergal margin and basal margin of T. kuroshioensis is more perpendicular (90°) or shaper while that of T. singaporensis is curved.
Tetraclita singaporensis
Tetraclita singaporensis Chan, Tsang & Chu, 2007: 52–53, figs 1–3.
Non-type material examined
Andaman Sea: 2 specimens, Phang-nga province, Takua Thung district, Na Tai beach, 16.V.2015, A. Pochai (BUU16.TS.TSG01-02).
Description
Peduncle absent; base membranous; shell purplish-dusky green with 4 plates (1 carina, 2 latus, 1 rostrum), parietes conical, plates inseparable, 5–6 rows of parietal tubes (parietes multiple tubiferous), external surface with deep and irregular longitudinal striations from apex to base and small radiating lines, internal surface of parietes smooth and white with greyish-green horizontal striations around aperture. External surface of operculum yellowish brown mixed with dusky green, internal surface of operculum dusky green-purplish and white around spur of the tergum. Scutum bigger than tergum, scutum triangular, short articular ridge-basal margin, external surface of scutum with horizontal striations, occludent margin of scutum with rough teeth. Tergum higher than wide, tergum with broad spur and not beaked, spur angle 30–35°, basi-scutal margin 148–150°. Mandible with 4 big teeth, 2nd and 3rd teeth consisting double teeth, 1st tooth with small spines, lower margin pectinate with 8 small teeth and obvious double bigger teeth at the edge; maxillule notched, two large setae above notch, 13–17 setae below notch; labrum with 4–5 large teeth on each side; cirri I possessing bidenate serrulate setae.
Tetraclita singaporensis collected from (BUU16.TC.TSG02) from Na Tai beach, Phang-nga. A. Dorsal and ventral view of external shell, B. External (left panel) and internal (right panel) view of tergum (upper panel) and scutum (lower panel), C. Lateral side showing external surface of shell, D. Close up on the external surface of shell, E–H. Light microscopy on mouthparts, E. Labrum, E. Close up on the teeth of the labrum, G. Mandible, G. Close up on the pectinated lower margin of mandible. E–H. Scale bars in µm.
Distribution
Tetracliata singaporensis has been reassigned the name from previously known as Tetraclita squamosa, which were collected from Singapore. Hence, the distribution of this species is firstly marked at Singapore, Indo-West Pacific region (
Remarks
Tetraclita singaporensis differs from Tetraclita squamosa in that it has tergum without beak and broader spur, and scutum with short articular ridge-basal margin.
Tetraclita squamosa
Balanus squamosa Bruguiére, 1789: 170.
Lepas porosa Gmelin, 1791: 3212.
Tetraclita
porosa
var.
viridis
:
Tetraclita
squamosa
:
Tetraclita
squamosa
squamosa
:
Tetraclita
squamosa
forma
viridis
:
Tetraclita porosa perfecta Nilsson-Cantell, 1921: 364.
Tetraclita
squamosa
:
Non-type material examined
Gulf of Thailand: 2 specimens, Nakhon Si Thammarat province, Sichon district, Hin Ngam beach, 04.VII.2015, A. Pochai (BUU16.TC.TSS01-02).
Description
Peduncle absent; base membranous; shell green mixed with brownish grey, shell with 4 plates (1 carina, 2 latus, 1 rostrum); parietes conical, plate fused, inseparable, 8 rows of parietal tubes (parietes multiple tubiferous), external surface with longitudinal lines from base to apex, internal surface of parietes smooth and white with purplish grey striations close to aperture, External surface of operculum brownish grey, internal surface of operculum purplish grey. Scutum larger than tergum, scutum triangular, long articular ridge-basal margin, external surface of scutum with horizontal striations, occludent margin of scutum with very shallow teeth; tergum higher than wide, basi-scutal margin 158–160°, tergum apex obviously beaked, tergum with spur long and sharp, spur angle 25°. Mandible with 4 big teeth, 1st tooth with three small spines, lower margin pectinate; maxillule notched, two large setae above notch, 11 big setae below notch and some smaller setae at the edge; labrum with 4 large teeth on each side; cirri I possessing bidenate serrulate setae.
Tetraclita squamosa collected from Hin Ngam beach, Nakhon Si Thammarat (BUU16.TC.TS01). A. Dorsal and ventral view of external shell, B. External (left panel) and internal (right panel) view of tergum (upper panel) and scutum (lower panel), C. External (upper panel) and internal (lower panel) view of shell plates, D. Close up on external surface of shell, E–H. Light microscopy on mouthparts, E. Labrum, E. Close up on the teeth of the labrum, G. Mandible, G. Close up on the pectinated lower margin of mandible. E–H. Scale bars in µm. Abbreviations: c, carina; l, latus; r, rostrum.
Distribution
Tetraclita squamosa is widespread in tropical and subtropical waters from West Africa, the Indo-Pacific, the Indian Ocean, Australia, Indonesia and Singapore (
Remark
As described in
Superfamily Balanoidea Leach, 1817
Family Balanidae Leach, 1817
Subfamily Amphibalaninae Pitombo, 2004
Amphibalanus
Type species
Amphibalanus amphitrite (Darwin, 1854)
1 genus, 2 species recorded: Amphibalanus amphitrite (Darwin, 1854) and Amphibalanus reticulatus (Utinomi, 1967).
Amphibalanus amphitrite
Balanus amphitrite var. communis Darwin, 1854: 240 (in part).
Balanus
amphitrite
Weltner, 1897: 264;
Balanus
amphitrite
communis
:
Balanus
amphitrite
hawaiiensis
:
Amphibalanus
amphitrite
:
Non-type material examined
Andaman Sea: 2 specimens, Phang-nga province, Khura Buri district, Ao Khoei beach, 30.VII.2015, A. Pochai (BUU16.BN.AA01-02). 4 specimens, Phang-nga province, Takua Thung district, Na Tai beach, 16.V.2015, A. Pochai (BUU16.BN.AA03-06). 4 specimens, Phuket province, Mueang Phuket district, Ao Yon beach, 15.VII.2015, A. Pochai (BUU16.BN.AA07-10). 3 specimens, Phuket province, Mueang Phuket district, Panwa beach, 16.VII.2015, S. Khachonpisitsak (BUU16.BN.AA11-13). 4 specimens, Phuket province, Katu district, Kalim beach, 15.VII.2015, A. Pochai (BUU16.BN.AA14-17).
Gulf of Thailand: 2 specimens, Nakhon Si Thammarat province, Sichon district, Hin Ngam beach, 09.VIII.2015, A. Pochai (BUU16.BN.AA18-19). 4 specimens, Prachuap Khiri Khan province, Bang Saphan district, Ban Krut beach, 06.IX.2015, A. Pochai (BUU16.BN.AA20-23). 2 specimens, Chon Buri province, Ko Si Chang district, Ko Kham Yai beach, 05.VII.2015, S. Khachonpisitsak (BUU16.BN.AA24-25). 4 specimens, Chon Buri province, Si Racha district, Si Racha beach, 04.VII.2015, A. Pochai (BUU16.BN.AA26-29). 3 specimens, Chon Buri province, Mueang Chon Buri district, Khao Sam Muk beach, 05.VII.2015, A. Pochai (BUU16.BN.AA30-32).
Description
Peduncle absent; base calcareous. Shell white-pale pink with 6 plates (1 carina, 2 carinal latus, 2 latus, 1 rostrum); single rows of parietal tubes (parietes single tubiferous) with transverse septa; radii solid. External surface with purple longitudinal striations from apex to base (3–4 lines per plate) without horizontal striation, transverse teeth on suture edges with denticles on lower regions, internal surface of parietes grey with black horizontal striations close to operculum. External surface of operculum brownish grey, internal surface of operculum grey-white. Scutum bigger than tergum, scutum triangular, external surface of scutum with curved striations; tergum spur board with growth lines.
Amphibalanus amphitrite collected from Khao Sam Muk beach, Chon Buri (BUU16.BA.AA30). A. Dorsal and ventral view of external shell, B. External (left panel) and internal (right panel) view of tergum (upper panel) and scutum (lower panel), C. External (upper panel) and internal (lower panel) view of shell plates. Abbreviations: c, carina; cl, carinal latus; l, latus; r, rostrum.
Distribution
Amphibalanus amphitrite is a common fouling barnacle and cosmopolitan species distributed along intertidal zones of coastlines in both the Gulf of Thailand and the Andaman Sea. It was found in all stations examined. The settlement patterns are various (e.g. rocks, shells of oyster and green mussels, concrete walls of bridges and harbors, offshore vessels, dock pilling, and mooring robes). In previous records, this species distributes worldwide in both tropical and temperate regions including the Indo-West Pacific, and Western Australia (
Remark
The morphology of Amphibalanus amphitrite is variable from diverse habitats worldwide. Shells exposed and eroded by heavy wave action showed no purple stripes on the external surface. The molecular analysis has confirmed its genetic differentiation which might be due to local adaptation and geographical isolation (
Amphibalanus reticulatus
Balanus amphitrite var. communis Darwin, 1854: 240, pl. 5, figs. 2e, h, l [type locality: Tachitgatani, Tanabe Bay, Japan].
Balanus
amphitrite
communis
:
Balanus
reticulatus
:
Non-type material examined
Andaman Sea: 2 specimens, Phang-nga province, Takua Thung district, Na Tai beach, 16.V.2015, A. Pochai (BUU16.BN.AR01-02).
Gulf of Thailand: 3 specimens, Chon Buri province, Si Racha district, Si Racha beach, 04.VII.2015, A. Pochai (BUU16.BN.AR03-05). 3 specimens, Chon Buri province, Mueang Chon Buri district, Khao Sam Muk beach, 05.VII.2015, A. Pochai (BUU16.BN.AR06-08). 3 specimens, Chon Buri province, Ko Si Chang district, Ko Kham Yai beach, 05.VII.2015, S. Khachonpisitsak (BUU16.BN.AR09-11).
Description
Peduncle absent; base calcareous. Shell white-pale pink and orange with 6 plates (1 carina, 2 carinal latus, 2 latus, 1 rostrum); single rows of parietal tubes (parietes single tubiferous) with transverse septa; radii solid. External surface with longitudinal and horizontal striations, transverse teeth on suture edges with denticles on lower regions, internal surface of parietes white. External surface of operculum white-pale pink and orange with striations in both tergum and scutum, internal surface of operculum white. Scutum bigger than tergum, scutum triangular; tergum spur sharp with growth lines.
Amphibalanus reticulatus collected from Si Racha beach, Chon Buri (BUU16.BN.AR01, A; BUU16.BN.AR 03, B & C). A. Dorsal and ventral view of external shell, B. External (left panel) and internal (right panel) view of tergum (upper panel) and scutum (lower panel), C. External (upper panel) and internal (lower panel) view of shell plates. Abbreviations: c, carina; cl, carinal latus; l, latus; r, rostrum.
Distribution
Amphibalanus reticulatus is widely distributed from Japan, the Indo-West Pacific to Australia, of which the latter is considered as an introduced species carried by ship transport (
Remark
Amphibalanus reticulatus exhibits clear vertical and horizontal striations while Amphibalanus amphitrite shows only vertical purple striation in all shell plates. In addition, the shapes of shell of A. reticulatus is more columnar than that of A. amphitrite, which might be due to elongation of parietes in response to crowding when growing as colonies. On all examined stations, distinct distribution and settlement between A. amphitrite and A. reticulatus can be noticed, in that A. amphitrite were found in almost all kinds of substrates but A. reticulatus preferred its attachment on shells which obviously did not live along the rocky shores and it might probably inhabit the deeper areas of the sea and were occasionally carried away into the shores by wave action.
Subfamily Megabalaninae Newman, 1979
Megabalanus
Type species
Megabalanus tintinnabulum (Linnaeus, 1758)
1 genus, 1 species recorded: Megabalanus tintinnabulum Linnaeus, 1758.
Megabalanus tintinnabulum
Lepas tintinnabulum Linnaeus, 1758: 668.
Balanus
tintinnabulum
:
Lepas tintinnabulum Wood, 1815: 38, pl. 6, figs. 1, 2.
Balanus
tintinnabulum
tintinnabulum
:
Balanus tintinnabulum var. tintinnabulum : Oliveira 1941: 11, text-fig. 1, pl. 2, figs. 1, 2, pl. 4, fig. 1, pl. 5 fig. 3, pl. 8, fig. 6.
Megabalanus
tintinnabulum
:
Non-type material examined
Andaman Sea: 3 specimens, Phang-nga province, Takua Thung district, Na Tai beach, 16.V.2015, A. Pochai (BUU16.BN.MT01-03).
Description
Peduncle absent; base calcareous. Shell cylindric or conic with 6 plates (1 carina, 2 carinal latus, 2 latus, 1 rostrum); parietes reddish to brownish red usually with longitudinal striations on external surface, parietes not prominently ribbed and rather smooth, irregular shape of parietal tubes (parietes tubiferous), sutural edges of radii with regular denticles, radii wide with horizontally striated, radii tubiferous; internal surface of parietes pale-purple with horizontal greyish violet striations around aperture. Orifice subcircular to rhombus. External surface of operculum white-pale pink and orange with prominent growth ridges in both tergums and scutums, internal surface of operculum white. Scutum bigger than tergum, scutum triangular, adductor ridge of scutum prominent; tergum with spur, spur furrow of tergum closed, scutal margin denticulate.
Megabalanus tintinnabulum collected from Na Tai beach, Phang-nga (BUU16.BN.MT01). A. Dorsal and ventral view of external shell, B. External (left panel) and internal (right panel) view of tergum (upper panel) and scutum (lower panel), C. External (upper panel) and internal (lower panel) view of shell plates. Abbreviations: c, carina; cl, carinal latus; l, latus; r, rostrum.
Distribution
Megabalanus tintinnabulum is widely distributed across almost all continents and is a well-known cosmopolitan fouling species. It was previously found in French Guiana, the United States, Australia, Mexico, Ecuador, Kuwait, Saudi Arabia, Sweden, France, Netherlands, Singapore, Indonesia and India (
Remarks
Megabalanus tintinnabulum has relatively larger shell plates than those of Amphibalanus. All three examined species (M. tintinnabulum, A. amphitrite and A. reticulatus) in family Balanidae have opercular valves with prominent growth ridges horizontally, and tergum with a clear spur. The coloration among these three species is easily distinguishable, in that purplish longitudinal striations presenting A. amphitrite, vertical and longitudinal red-orange striations with orange-pale pink background presenting A. reticulatus and brownish red surface with some irregular and unclear longitudinal stripes presenting M. tintinnabulum.
Identification key
| 1a | Shell conical to low conical with 4 plates with distinct parietes or shell with 4 plates with indistinct parietes or fused parietes, parietes multi-tubiferous | 2 |
| 1b | Shell with 6 plates with distinct parietes | 5 |
| 2a | Shell low conical, parietes discrete, summit of radii oblique, orifice pentagonal and wide, external surface of shell with deep longitudinal striations | Newmanella spinosus Chan & Cheang, 2016 |
| 2b | Shell conical, parietes not discrete, summit of radii horizontal, orifice circular or oval and small | 3 |
| 3a | Shell green, external surface of shell with longitudinal striation, shell plates separable, tergum with obvious beak and tergum with sharp and narrow spur | Tetraclita squamosa (Bruguiere, 1789) |
| 3b | Shell greyish black, external surface of shell with mosaic scale-like, plates inseparable, tergum without beak and with broad spur | 4 |
| 4a | Angle between basal margin and tergal margin of scutum is almost perpendicular | Tetraclita kuroshioensis Chan, Tsang & Chu, 2007 |
| 4b | Angle between basal margin and tergal margin of scutum is curved | Tetraclita singaporensis Chan, Tsang & Chu, 2007 |
| 5a | Parietes solid | 6 |
| 5b | Parietes tubiferous | 8 |
| 6a | Body length 10–30 mm, gigantic appearance | Euraphia hembeli Conrad, 1837 |
| 6b | Body length 3–10 mm, tergum with 3–4 lateral depressor crests | 7 |
| 7a | Mandible with four teeth, cirri I with conical spines, cirri II with multi-cuspidate setae and basal guard, articulation of opercular valves deep (shape of articulation similar to jigsaw-shaped) | Chthamalus malayensis Pilsbry, 1916 |
| 7b | Mandible with three teeth and 11 smaller setae at the lower margin, articulation of opercular valves shallow (shape of articulation from outside view similar to bird beak) | Euraphia depressa (Poli, 1795) |
| 8a | Parietal tubes single row and irregular shaped, shell with irregular and deep longitudinal striations, shell purplish white | Megabalanus tintinnabulum (Linnaeus, 1758) |
| 8b | Parietal tubes single row and uniform | 9 |
| 9a | External surface with purple longitudinal striations from apex to base against white surface | Amphibalanus amphitrite (Darwin, 1854) |
| 9b | External surface with shall longitudinal and horizontal striations, shell white-pale pink and orange | Amphibalanus reticulatus (Utinomi, 1967) |
Discussion
In the present study, we examine geographical distribution of sessile acorn barnacles along Thai Peninsular coastal areas including the Gulf of Thailand and the Andaman Sea. So far, there has been a lack of information regarding the diversity of sessilian Thoracican barnacles in Thailand. Hence, we attempt to generate a checklist to understand the species diversification and how they distribute on intertidal rocky shores and sandy shores along the coast of Thailand. At least ten different forms of acorn barnacles were diagnosed so far that are classified into 6 genera and 3 families (Chthamalidae, Tetraclitidae and Balanidae), which can be distinguished based on their external shell morphology, including pattern of parietes, opercular plates, and arthropodal characters as described in previous literatures (
Our study also shows that the numbers of species found in the Andaman Sea (8 species) are more than those found in the Gulf of Thailand (6 species). At Na Tai station located in the Andaman Sea, up to 8 species (6 genera and 3 families) were recorded. Four of these 8 species were found only at this station including Newmanella spinosus, Euraphia hembeli, Megabalanus tintinnabulum and Tetraclita singaporensis. In other examined stations, only 2–3 species could be found, and most of them were of the genus Amphibalanus, Tetraclita, and Chthamalus. The differences in species abundance between two coastlines might probably due to the past history of the barnacle colonization. It has been shown in
In addition, we found five new records identified as Newmanella spinosus, Euraphia depressa and Euraphia hembeli, Tetraclita singaporensis, and Tetraclita kuroshioensis on which the presence of these species in Thailand has not been mentioned in any literatures. N. spinosus, E. hembeli and T. singaporensis can only be seen at Na Tai station, Phang-nga province while E. depressa is specific to Khao Sam Muk, Chon Buri province. However, we cannot rule out the possibility of their presences in other places and more intensive field surveys covering all provinces along Thailand's coasts are required.
Recently, there are 26 species in the genus Chthamalus (
According to a field survey on water quality and metal contamination of both coastal regions of Thailand, the Andaman Sea is still in a good condition compared to the Gulf of Thailand. On the other hand, habitat degradation along the Gulf of Thailand is much more severe and the number of species of these sessile arthropods has been declining dramatically over the last 20 years due to high amount of water pollution. For example, along Chon Buri’s coast around 20 years ago, at least five species were commonly seen along rocky shores of the now developing centrum area. Recently, however, only Amphibalanus amphitrite have been able to tolerate severe human activities and even in some sites there are no more barnacles on rocky shores. This might be because the local communities have been releasing non-treated waste water directly into the sea (personal communication and unpublished report (1996): Department of Biology, Faculty of Science, Burapha University). Hence, the richness of barnacle species can also be used to indirectly monitor the conditions of sea water.
Taken together, we demonstrate a clearer view of diversity for acorn barnacles from various localities in Thailand. This study shows at least 10 species of barnacles, in total, exist along Thai coast regions. Future works with more sampling sites and further in-depth investigations using SEM and molecular approaches with the help of phylogenetic analysis will provide a much better view especially of the history of barnacles and intraspecific variation between sessile crustaceans and that may reveal new barnacle species inhabiting Thailand.
Acknowledgement
This work was supported by Grant for Graduate Student 2015, Faculty of Science, Burapha University, Thailand. We would like to thank Assistant Professor Dr. Chuta Boonphakdee, Assistant Professor Pongrat Dumrongrojwattana, Mr. Rungwit Chaijirawong, Mr. Santi Suanla and Ms. Salisa Nithikulthananan for imaging assistance and Mr. Robert Luke for reading through a draft of the manuscript.
References
- Achituv Y, Safriel UN (1980) Euraphia depressa (Poli) (CrustaceaCirripedia), A recent Mediterranean colonizer of the Suez canal. Bulletin of Marine Science 30(3): 724–726.
- Barnard KH (1924) Contributions to the crustacean fauna of South Africa – 7. Cirripedia. Annals of the South Africa Museum 20: 1–103.
- Barrett PH, Freeman RB (2016) The Works of Charles Darwin: Vol 12: A Monograph on the sub-class Cirripedia (1854), Vol II. Routledge.
- Borradaile LA (1900) On some crustacean from the South Pacific. Part 5. Arthrostracans and barnacles. Proceedings of the Zoological Society of London 1900: 795–799.
- Brickner I, Høeg JT (2010) Antennular specialization in cyprids of coral-associated barnacles. Journal of Experimental Marine Biology and Ecology 392: 115–124. https://doi.org/10.1016/j.jembe.2010.04.015
- Brickner I, Loya Y, Achituv Y (2010) Diverse life strategies in two coral-inhabiting barnacles (Pyrgomatidae) occupying the same host (Cyphastrea chalcidicum), in the northern Gulf of Eilat. Journal of Experimental Marine Biology and Ecology 392: 220–227. https://doi.org/10.1016/j.jembe.2010.04.022
- Broch H (1922) Papers from Dr. Th. Mortensen’s Pacific Expedition 1914–1916, X. Studies on Pacific cirripedes. Videnskabelige Meddelelser fra Dansk Naturhistorisk Forening i Kobenhavn 73: 251–358.
- Broch H (1931) Papers from Dr. Th. Mortensen’s Pacific Expedition 1914–1916, LVI. Indomalayan Cirripedi. Videnskabelige Meddelelser fra Dansk Naturhistorisk Forening i Kobenhavn 91: 1–146.
- Bruguiére M (1789) Encyclopedie methodique. Historie naturelle des Vers. 1, 158–173.
- Carlton JT, Newman WA, Pitombo FB (2011) Barnacle invasions: Introduced, cryptogenic, and range expanding Cirripedia of North and South America. In: Galil BS, Clark PF, Carlton JT (Eds) In the Wrong Place – Alien Marine Crustaceans: Distribution, Biology and Impacts. Dordrecht, SpringerSeries in Invasion Ecology XVI: 159–214. https://doi.org/10.1007/978-94-007-0591-3-5
- Chan BKK (2001) Studies on Tetraclita squamosa and Tetraclita japonica (Cirripedia: Thoracica) I: adult morphology. Journal of Crustacean Biology 21: 616–630. https://doi.org/10.1651/C-2350
- Chan BKK, Tsang LM, Chu KH (2007a) Cryptic diversity of the Tetraclita squamosa complex (Crustacea: Cirripedia) in Asia: description of a new species from Singapore. Zoological Studies 46(1): 46–56.
- Chan BKK, Tsang LM, Chu KH (2007b) Morphological and genetic differentiation of the acorn barnacle Tetraclita squamosa (Crustacea, Cirripedia) in East Asia and description of a new species of Tetraclita. Zoological Scripta 36: 79–91. https://doi.org/10.1111/j.1463-6409.2007.00260.x
- Chan BKK, Hsu CH, Southward AJ (2008) Morphological variation and biogeography of an insular intertidal barnacle Hexechamaesipho pilsbryi (Crustacea: Cirripedia) in the Western Pacific. Bulletin of Marine Science 83(2): 315–328.
- Chan BKK, Prabowo RE, Lee K-S, Lee K-H (2009) Crustacean Fauna of Taiwan: Barnacles, Volume 1-Cirripedia: Thoracica excluding the Pyrgomatidae and Acastinae. National Taiwan Ocean University, Keelung.
- Chan BKK, Cheang CC (2016) First discovery of a new species of Newmanella Ross, 1969 (Balanomorpha: Tetraclitidae) in the western Pacific, with a note on the new status of Neonrosella Jones, 2010. Zootaxa 4098(2): 201–226. https://doi.org/10.11646/zootaxa.4098.2.1
- Chen HN, Tsang LM, Chong VC, Chan BKK (2014) Worldwide genetic differentiation in the common fouling barnacle, Amphibalanus amphitrite. Biofouling 30: 1067–1078. https://doi.org/10.1080/08927014.2014.967232
- Chen YY, Lin HC, Chan BKK (2012) Description of a new species of coral-inhabiting barnacle, Darwiniella angularis sp. n. (Cirripedia, Pyrgomatidae) from Taiwan. ZooKeys 214: 43–74. https://doi.org/10.3897/zookeys.214.3291
- Conrad TA (1837) Descriptions of new marine shells from upper California, collected by Thomas Nuttal, Esq. Journal of the Academy of Natural Sciences of Philadelphia (Series 1) 7: 227–268.
- Crisp D, Southward AJ, Southward E (1981) On the distribution of the intertidal barnacles Chthamalus stellatus, Chthamalus montagui and Euraphia depressa. Journal of the Marine Biological Association UK 61: 359–380. https://doi.org/10.1017/S0025315400047007
- Daniel A (1956) The cirripedes of the Madras coast. Bulletin of the Madras Government Museum 6(2): 1–40.
- Darwin C (1854) A monograph on the sub-class Cirripedia with figures of all species. The Balanidae, Verrucidae, 684 pp.
- Davadie C (1963) Etude des Balanes fossiles d’Europe et d’Afrique. Systématique et structure des balanes fossiles d’Europe et d’Arique, 146 pp.
- de Oliveira LPH (1941) Contribuicao ao conhecimento dos crustaceos do Rio de Janeiro. Sub-ordern “Balanomorphe” (Cirripedia: Thoracica). Memorias do Instituto Oswaldo Cruz 36(1): 1–31.
- Dong Y, Chen Y, Cai R (1980) Preliminary study on the Chinese cirripedian fauna (Crustacea). Acta Oceanologica Sinica 2: 124–131.
- Frith DW, Tantanasiriwong R, Bhatia O (1976) Zonation and abundance of macrofauna on a mangrove shore, Phuket Island. Phuket Marine Biological Center Research Bulletin 10: 37.
- Gmelin JF (1791) Systematic Naturae, 3212.
- Gruvel A (1905) Monographie des Cirrhipèdes ou thecostracés, 472 pp.
- Hayashi R (2013) A checklist of turtle and whale barnacles (Cirripedia: Thoracica: Coronuloidea). Journal of the Marine Biological Association of the United Kingdom 93(1): 143–182. https://doi.org/10.1017/S0025315412000847
- Hayashi R, Chan BKK (2015) New records of the tetraclitid barnacle Tesseropora alba (Cirripedia: Thoracica: Tetraclitoidea) in the Pacific waters of Taiwan and Okinawa. Species Diversity 20(2): 183–189. https://doi.org/10.12782/sd.20.2.183
- Hawkins SJ, Moore PJ, Burrows MT, Poloczanska E, Mieszkowska N, Herbert RJH, Jenkins SR, Thompson RC, Genner MJ, Southward AJ (2008) Complex interactions in a rapidly changing world: Responses of rocky shore communities to recent climate change. Climate Research 37: 123–133. https://doi.org/10.3354/cr00768
- Helmuth BST, Mieszkowska N, Moore P, Hawkins SJ (2006) Living on the edges of two changing world: Forecasting the responses of rocky intertidal ecosystems to climate change. Annual Review of Ecology, Evolution and Systematics 37: 373–404. https://doi.org/10.1146/annurev.ecolsys.37.091305.110149
- Henry DP (1957) Some littoral barnacles from the Tuamotu, Marshall, and Caroline Islands. Proceedings of the United States national Museum 107(3381): 25–38. https://doi.org/10.5479/si.00963801.107-3381.25
- Henry DP, McLaughlin PA (1975) The barnacles of the Balanus amphitrite complex (Cirripedia, Thoracica). Zoologische Verhandelingen 141: 1–254.
- Henry DP, McLaughlin PA (1986) The recent species of Megabalanus (Cirripedia, Balanomorpha) with special emphasis on Balanus tintinnabulum (Linnaeus) sensu lato. Zoologische Verhandelingen 235: 1-69.
- Hiro F (1936) Report on the cirripedia collected in the Malayan waters by the ship ‘Zuiho-maru’. Japanese Journal of Zoology 6(4): 621–636.
- Hiro F (1937) Studies on cirripedian fauna of Japan II. Cirripeds found in the vicinity of the Seto marine Biological Laboratory. Memoirs of the College of Science, Kyoto Imperial University, Series B 12: 385–478.
- Hiro F (1938) On the Japanese forms of Balanus amphitrite Darwin. Zoological Magazine (Tokyo) 50: 299–313.
- Hiro F (1939) Studies on the cirripedian fauna of Japan. IV. Cirripeds of Formosa (Taiwan), with some geographical and ecological remarks. Memoirs of the College of Science, Kyoto Imperial University, Series B 15: 245–284.
- Hoek PPC (1913) Cirripedia of the Siboga-Expedition. Siboga-Expeditie Reports 31: 129–275.
- Holm ER (2012) Barnacles and biofouling. Integrative and Comparative Biology 52(3): 348–55. https://doi.org/10.1093/icb/ics042
- Holthuis LB, Heerebout GR (1972) Vondsten van de zeepok Balanus tintinnabulum (Linnaeus, 1758) in Nederland. Bijdragen tot de Faunistiek van Nederland. II. Zoologische Bijdragen, Leiden13: 24–31.
- Høeg JT, Møller OS (2006) When similar beginnings lead to different ends: Constraints and diversity in cirripede larval development. Invertebrate Reproduction & Development 49: 125–142. https://doi.org/10.1080/07924259.2006.9652204
- Jones DS, Hewitt MA, Sampey A (2000) A checklist of the Cirripedia of the South China Sea. The Raffles Bulletin of Zoology 8: 233–307.
- Jones DS (2004) Barnacles (Cirripedia: Thoracica) of the Dampier Archipelago, Western Australia. Records of the Western Australian Museum Supplement 66: 121–154.
- Karande AA, Palekar VC (1963) On a shore barnacle Chthamalus malayensis Pilsbry from Bombay, (India). Annals and Magazine of Natural History, series 136: 231–234.
- Kim MH, Yamaguchi T (1996) Larval development and phylogenetic relationship between Chthamalus challengeri and Euraphia pilsbryi (Subclass Cirripedia, Suborder Balanomorpha, Family Chthamalidae). Marine Fouling 12: 1–23.
- Kolosváry GV (1943) CirripediaThoracica in der Sammlung des Ungarischen National-Museums. Annales Historico-Naturales Musei Nationalis. Hungarici 36: 67–120.
- Krüger DP (1911) Beitrage zur Cirripedienfauna Ostasien. Beitrage zur Naturgeschichte Ostasiens herausgegeben von. F. Doflein. Konglige Bayerische Akademie der Wissenschaften, Munich Mathematische-physikalische Klasse. Abhandlungen Supplement Band 2: 1–72.
- Lacombe D, Rangel EF (1978) Cirripédios de Arraial do Cabo, Cabo Frio. Publicações do Intituto de Pesquisas da Marina 129: 1–12.
- Limpsaichol P, Khokiattiwong S, Bussarawit N (1991) Water quality of the Andaman Sea coast of Thailand. Technical paper. Phuket Marine Biological Center, Phuket, Thailand.
- Linnaeus C (1758) Systema Naturae. Homiae. Editio Decima, ReformataVolume 1, 824 pp.
- Lozano-Cortés DF, Londoño-Cruz E (2013) Checklist of barnacles (Crustacea; Cirripedia: Thoracica) from the Colombian Pacific. Marine Biodiversity 43(4): 463–471. https://doi.org/10.1007/s12526-013-0175-2
- Martin JW, Olesen J, Høeg JT (2014) Atlas of Crustacean Larvae. Johns Hopkins University Press, Baltimore.
- Maruzzo D, Aldred N, Clare AS, Høeg JT (2012) Metamorphosis in the Cirripede Crustacean Balanus amphitrite. PLoS ONE 7: e37408. https://doi.org/10.1371/journal.pone.0037408
- Miller KM, Blower SM (1989) Comparison of larval and adult stages of Chthamalus dalli and Chthamalus fissus (Cirripedia: Thoracica). Journal of Crustacean Biology 9: 242–256. https://doi.org/10.2307/1548504
- Molnar JL, Gamboa RL, Revenga C, Spalding MD (2008) Assessing the global threat of invasive species to marine biodiversity. Frontiers in Ecology and the Environment 6(9): 485–492. https://doi.org/10.1890/070064
- Newman WA, Ross A (1976) Revision of the balanomorph barnacles, including a catalog of the species. Memoirs of the San Diego Society of Natural History 9: 1–108.
- Nilsson-Cantell CA (1921) Cirripeden-Studien. Zur Kenntnis der Biologie, Antomie und Systematic dieser Gruppe. Zoologiska Bidrag Fran Uppsala 7: 75–390.
- Pilsbry HA (1907) Hawaiian cirripedia. Bulletin of the Bureau of Fisheries, Washington 26: 181–190.
- Pilsbry HA (1916) The sessile barnacles (Cirripedia) contained in the collections of the US National Museum: including a monograph of the American species. Bulletin of the United States National Museum 93: 1–366. https://doi.org/10.5479/si.03629236.93.1
- Pilsbry HA (1928) Littoral barnacles of the Hawaiian islands and Japan. Proceedings of the Academy of Natural Science of Philadelphia 79: 305–317.
- Pitombo FB (2004) Phylogenetic analysis of the Balanidae (Cirripedia, Balanomorpha). Zoological Scripta 33(3): 261–276. https://doi.org/10.1111/j.0300-3256.2004.00145.x
- Poli GS (1791) Testacea utriusque Siciliae eorumque historia et anatome, 1. Parmae. https://doi.org/10.5962/bhl.title.79042
- Pollution Control Department (2001) A report of sea water of Gulf of Thailand. Ministry of Natural Resources and Environment, Bangkok, Thailand.
- Pope EC (1965) A review of Australian and some Indomalayan Chthamalidae (Crustacea, Cirripedia). Proceedings of the Linnean Society of New South Wales 90: 10–77.
- Rawangkul S, Angsupanich S, Panitchart S (1995) Preliminary study of barnacles damaging the mangrove plantation Rhizophora mucronata at Tha Phae canal, Nakorn Si Thammarat. In: The ninth national seminar on mangrove ecology, mangrove conservation for Thai society in the next decade.National Research Council of Thailand Bangkok. Paper No. III-06.
- Ren X (1984) Studies on Chinese Cirripedia (Crustacea). III. Family Chthamalidae. Studia Marina Sinica 22: 145–163.
- Ren X, Lui R (1979) Studies on Chinese Cirripedia (Crustacea) II. Family Tetraclitidae. Oceanologia et Limnologia Sinica 10(4): 338–353.
- Ross A, Perreault RT (1999) Revision of the Tetraclitellinae and description of a new species of Newmanella Ross from the tropical Western-Atlantic Ocean (Cirripedia: Tetraclitoidea). Sessile organisms 15(2): 1–8. https://doi.org/10.4282/sosj.15.2_1
- Rossel NC (1972) Some barnacles (Cirripedia, Thoracica) of Puerto Galera found in the vicinity of the U.P. Marine Biological Laboratory. Natural and Applied Science Bulletin, Philippines 24: 143–285.
- Santhakumaran LN, Sawant SG (1991) Biodeterioration of mangrove vegetation by marine organisms along Indian coast - an annotated bibliography, Wood Biodegradation Division (Marine) 403004: 48.
- Sophia Rani S, Pmbhu S, Przyadharshini S (2010) Infestation of barnacle (Balanus amphitrite) in the mangrove environment. World Journal of Fish and Marine Sciences 2(4): 307–310.
- Southward AJ (1964) On the European species of Chthamalus stellatus (Cirripedia). Crustaceana 6: 241–254. https://doi.org/10.1163/156854064X00010
- Southward AJ, Burton RS, Coles SL, Dando PR, DeFelice R, Hoover J, Parnell PE, Yamaguchi T, Newman WA (1998) Invasion of Hawaiian shores by an Atlantic barnacle. Marine Ecology Progress Series 165: 119–126. https://doi.org/10.3354/meps165119
- Southward AJ, Newman WA (2003) A review of some common Indo-Malayan and western Pacific species of Chthamalus barnacles (Crustacea: Cirripedia). Journal of the Marine Biological Association of the United Kingdom 83: 797–812. https://doi.org/10.1017/S0025315403007835h
- Stebbing TRR (1910) General catalogue of South African Crustacea. Annals of the South Africa Museum 6(4): 563–575.
- Stubbings HG (1964) Cirripedia from the Congo Estuary and adjacent coasts in the Musee Royal de l’Afrique Centrale, Tervuren, Belgium.
- Stubbings HG (1967) The Cirripedia fauna of tropical West African. Bulletin of the British Museum (Natural History), Zoology 15(6): 229–319.
- Thiyagarajan V, Venugopalan V, Subramoniam T, Nair K (1997) Description of the naupliar stages of Megabalanus tintinnabulum (Cirripedia: Balanidae). Journal of Crustacean Biology 17(2): 332–342. https://doi.org/10.2307/1549282
- Tsang LM, Chan BKK, Wu TH, Ng WC, Chatterjee T, Williams GA, Chu KH (2008) Population differentiation in the barnacle Chthamalus malayensis: postglacial colonization and recent connectivity across the Pacific and Indian Oceans. Marine Ecology Progress Series 364: 107–118. https://doi.org/10.3354/meps07476
- Tsang LM, Wu TH, Shih HT, Williams GA, Chu KH, Chan BKK (2012) Genetic and morphological differentiation of the Indo-West Pacific intertidal barnacle Chthamalus malayensis. Integrative and Comparative Biology 52: 388–409. https://doi.org/10.1093/icb/ics044
- Utinomi H (1954) Invertebrate fauna of the intertidal zone of the Tokara Islands. IX. Cirripedia. Publications of the Seto Marine Biological Laboratory 4: 17–26.
- Utinomi H (1956) Colored illustrations of seashore animals of Japan, 168 pp. [pls. 64, I-VII]
- Utinomi H (1959) Thoracic cirripeds from the environ of Banyuls. Vie Milieu 10: 379–399.
- Utinomi H (1960) On the world-wide dispersal of a Hawaiian barnacle, Balanus amphitrite hawaiiensis Broch. Pacific Science 14(1): 43–50.
- Utinomi H (1967) Comments on some new and already known cirripeds with emended taxa with special reference to the parietal structure. Publications of the Seto Marine Biology Laboratory 15: 199–237.
- Utinomi H (1968) Pelagic shelf and shallow-water cirripedia from the Indo-west pacific. Videnskabelige Meddelelser fra Dansk Naturhistorisk Forening i Kobenhavn 131: 161–186.
- Voris HK (2000) Maps of Pleistocene sea levels in Southeast Asia shorelines, river systems and time durations. Journal of Biogeography 27: 1153–1167. https://doi.org/10.1046/j.1365-2699.2000.00489.x
- Weltner W (1987) Verzeichnis der bisher beschriebenen recenten Cirripedienarten. Archiv für Naturgeschichte 1: 227–280.
- Wood W (1815) General conchology, or a description or shells arranged according to the Linnean System 1: 246 pp.
- Yamaguchi T (1987) Changes in the barnacle fauna since the Miocene and the infraspecific structure of Tetraclita in Japan (Cirripedia: Balanomorpha). Bulletin of Marine Science 41: 337–359.
- Yan Y, Chan BKK (2004) A new barnacle species from Hong Kong: Chthamalus neglectus sp. nov. (Cirripedia: Thoracica: Chthamalidae). Journal of the Marine Biological Association of the United Kingdom 84: 133–138. https://doi.org/10.1017/S0025315404008999h
- Yu MC, Kolbasov GA, Chan BKK (2016) A new species of sponge inhabiting barnacle Bryozobia (Archaeobalanidae, Bryozobiinae) in the West Pacific. ZooKeys 571: 1–20. https://doi.org/10.3897/zookeys.571.6894
- Zevina GB, Tarasov NI (1963) Cirripediathoracica of the mainland coasts of south-eastern Asia (Yellow, East and South China Seas). Trudy Instituta Okeanology 70: 76–100.
Supplementary materials
Arthropodal characters of Tetraclita kuroshioensis
Data type: species data
Explanation note: Tetraclita kuroshioensis collected from (BUU16.TC.TK01) from Na Tai beach, Phang-nga. A.-I. Light microscopy on arthropodal characters. A. Cirri I, B.-C. Close up on cirri I showing serrulate setae, D. Cirri II, E.-F. Close up on cirri II showing serrulate setae, G. Maxillule, H. Mandible, I. Labrum.
Arthropodal characters of Tetraclita singaporensis
Data type: species data
Explanation note: Tetraclita singaporensis collected from (BUU16.TC.TSG02) from Na Tai beach, Phang-nga. A.-I. Light microscopy on arthropodal characters. A. Cirri I, B.-C. Close up on cirri I showing serrulate setae, D. Cirri II, E.-F. Close up on cirri II showing serrulate setae, G. Maxillule, H. Mandible, I. Labrum.
Arthropodal characters of Tetraclita squamosa
Data type: species data
Explanation note: Tetraclita squamosa collected from Hin Ngam beach, Nakhon Si Thammarat (BUU16.TC.TS01). A.-I. Light microscopy on arthropodal characters. A. Cirri I, B.-C. Close up on cirri I showing serrulate setae, D. Cirri II, E.-F. Close up on cirri II showing serrulate setae, G. Maxillule, H. Mandible, I. Labrum.